Abstract
We describe herein histologic, immunohistochemical, and molecular findings and clinical manifestations of a rare case of an extremely well differentiated papillary thyroid carcinoma (EWD-PTC). Similarly, it is also difficult to diagnose follicular variant papillary thyroid carcinoma (FVPTC), whose diagnosis is still met with controversy. A recently reported entity of well-differentiated tumor of uncertain malignant potential (WDT-UMP) is added to the diagnostic spectrum harboring EWD-PTC and FVPTC. We report this case, because EWD-PTC is different from FVPTC in its papillary architecture, and also from WDT-UMP in its recurrence and metastatic pattern. These morphologically deceptive entities harbored diagnostic difficulties in the past because the diagnosis depended solely on histology. However, they are now diagnosed with more certainty by virtue of immunohistochemical and molecular studies. We experienced a case of EWD-PTC, which had been diagnosed as adenomatous hyperplasia 20 years ago and manifested recurrence with lymph node (LN) metastasis 7 years later. After another 7 years of follow-up, a new thyroid lesion had developed, diagnosed as FVPTC, with LN metastasis of EWD-PTC. One year later, the patient developed metastatic FVPTC in the skull. Immunohistochemically, the EWD-PTC was focally positive for CK19, negative for galectin-3, and focally negative for CD56. Molecular studies revealed BRAF-positivity and K-RAS negativity. The FVPTC in the left thyroid showed both BRAF and K-RAS negativity. In conclusion, EWD-PTC and FVPTC share similar histologic features, but they are different tumors with different molecular biologic and clinical manifestations. A large cohort of EWD-PTC should be included in further study.
Diagnosis of extremely well differentiated papillary thyroid carcinoma (EWD-PTC) is controversial, and has not been clearly defined. Similar to EWD-PTC, diagnosis of follicular variant papillary thyroid carcinoma (FVPTC) is also controversial. The diagnosis is not so problematic when its nuclear features are typical of papillary thyroid carcinoma (PTC), however, its interpretation becomes a diagnostic dilemma when the nuclear features are not as typical.12 Diagnosis of well differentiated tumors of uncertain malignant potential (WDT-UMP) has been suggested in cases showing that nuclear features are histologically inadequate for PTC and also clinical features inadequate for malignancy, thus avoiding overtreatment.34 We herein report a case showing histologic features similar to WDT-UMP, but with clinically malignant behavior. Insight understanding of such a deceptive case will enable pathologists to reach an accurate diagnosis and clinicians to decide the most appropriate treatment plan.
A 25-year-old man presented with a large solid and colloid mass measuring 10×7 cm in the right thyroid, which was diagnosed as adenomatous hyperplasia. Seven years later, he presented with a 4 cm-sized, suddenly growing mass in the right side of the neck, and completion right total thyroidectomy with lymph node (LN) dissection was done. The specimen consisted of 16 LNs with metastatic follicular thyroid lesions showing no definite nuclear features of PTC (Fig. 1A). Immunohistochemistry for CK19 (DAKO, Glostrup, Denmark) and Galectin-3 (NOVOCASTRA, Newcastle, UK) were done, and although their results were not supportive (Fig. 1B), the LN were diagnosed as metastatic PTC, considering the metastatic pattern. Radioactive iodine (RAI) therapy was not done because the left thyroid was preserved. Another seven years later, he developed a mass in the left thyroid, which was diagnosed as encapsulated FVPTC without capsular or vascular invasion (Fig. 1C), and metastatic LNs displaying same histology as the second presentation (Fig. 1F). Again, the FVPTC did not show typical nuclear features of PTC, and the immunohistochemistry results of CK19, Galectin-3, and p63 (NOVOCASTRA) were not supportive. However, immunohistochemistry for CD56 (ZYMED, South San Francisco, CA, USA) revealed loss of expression in the follicular cells, rendering the diagnosis of FVPTC (Fig. 1D and E). Immunohistochemical profile of the metastatic PTC in the LNs was the same as that of FVPTC (Fig. 1G and H).5 Preoperative thyroglobulin level was 321.85 ng/mL, which dropped to 185.82 ng/mL postoperatively (Table 1). The patient received RAI 150 mCi treatment, and the thyroglobulin level decreased to 49.0 ng/mL with thyroid stimulating hormone (TSH) suppression. Six months later, due to radiologically suspicious metastatic lesion in the frontal bone and left 5th rib with thyroglobulin level of 460.0 ng/mL, the patient underwent surgical removal of the skull lesion, diagnosed as metastatic FVPTC (Fig. 1I, J, and K) and received additional RAI 200 mCi treatment. Afterwards, the patient received palliative radiation on the skull and additional RAI 200 mCi treatments. Presently, no remarkable change in the activity of radioiodine uptake is seen in the bone, and the thyroglobulin level is 0.7 ng/mL with TSH suppression; thus, the patient is considered to be in well-controlled status (Table 1). We performed immunohistochemistry for CD31 (DAKO) and PPAR-γ (CELL SIGNALING, Danvers, MA, USA) for detecting vascular invasion of FVPTC, and molecular studies for BRAF and K-RAS in available specimen after the third presentation employing PNA clamping method (Panagene, Daejeon, Korea).6 When the FVPTC was thoroughly examined, no capsular invasion was identified, and no vascular invasion was seen on immunohistochemistry. Both the FVPTC and the LN metastasis showed wild type of K-RAS. In BRAF study, only the LN metastasis showed mutation, but the FVPTC and bone metastasis did not (Table 2).
Since the introduction of FVPTC, its diagnostic criteria have been established and settled over time, nevertheless, a wide spectrum of interobserver variability still exists even among thyroid experts,2 thus lowering diagnostic accuracy in cytology and intraoperative frozen sections.78 Adenomatous hyperplasia showing nuclear features reminiscent, but not quite diagnostic, of PTC have been most troublesome, and they were generally passed as adenomatous hyperplasia, because cellular atypia to some degree, increase in size, and recurrence are all possible in adenomatous hyperplasia. With this diagnostic dilemma, many ancillary diagnostic tools have been improvised to increase the diagnostic accuracy in FVPTC; immunohistochemistry for CK19, HBME1, and galectin-3 have been shown to be helpful in the diagnosis of PTC. However, the results have not been satisfactory. The loss of CD56 immunoreactivity in the follicular cells have been reported to be specific to PTC, and our experience indicates that the loss of CD56 immunoreactivity is relatively specific to conventional PTC as well as FVPTC.9 When our patient first presented with thyroid mass 20 years ago, the concept of FVPTC had not yet been introduced. Seven years later when the tumor metastasized to the LNs, the cytologic features were insufficient for the diagnosis of PTC. Moreover, the tumor showed negative immunohistochemistry for CK19, HBME1, and galectin-3. The diagnosis of LN metastasis was solely based on the metastatic pattern of the tumor. Such cases present diagnostic dilemma, and clinical evidence of malignancy is even more difficult to find if follow-up period is short. Many efforts have been spent for finding the most appropriate diagnosis for such cases, and resulted in the newly evolving diagnosis of thyroid tumors of uncertain malignant potential, reflecting the borderline clinical behavior. Recurrence or metastasis has not been found in such cases, although the longest follow-up period was 9 years. No molecular biologic abnormality was found.10 Our case shows relatively typical clinical features of PTC and FVPTC, with two episodes of LN metastasis, newly developed lesion of FVPTC, and bone metastasis during the 15 years of follow-up.
In the present case, the tumor showed BRAF expression in areas that are histologically different from FVPTC. These features suggested that the newly developed FVPTC and the pre-existing tumor were essentially different tumors. Furthermore, although the features of the LN metastasis and BRAF expression were compatible with conventional PTC, the histologic features were not. Therefore, the diagnosis of WDT-UMP is thought to be inadequate, and we suggested the diagnosis of EWD-PTC, stressing histologic features different from conventional PTC and excluding the possibility of underdiagnosis at the same time.
Figures and Tables
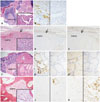 | Fig. 1Neck lymph node (LN) shows metastatic extremely well differentiated papillary thyroid carcinoma (EWD-PTC) (A, H-E stain, ×12.5 and inset ×400) with focal positivity of CK19 (B, ×400) in the first metastatic lesion. The left thyroid shows follicular variant papillary thyroid carcinoma (FVPTC) without capsular (arrow) invasion (C, H-E stain, ×40 and inset ×400) with focal positivity of CK19 (D, ×40) and loss of CD56 (E, ×40) in second episode. The LN also shows metastatic EWD-PTC (F, H-E stain, ×12.5 and inset ×400) with focal positivity of CK19 (G, ×400) and CD56 (H, ×400) in the second metastatic lesion. The skull lesion shows metastatic FVPTC (I, H-E stain, ×100 and inset ×400) with focal positivity of CK19 (J, ×400) and CD56 (K, ×400). |
Table 1
Summary of Biochemical Data

Table 2
Summary of Histopathologic Findings, Immunohistochemistry and Molecular Study

ACKNOWLEDGEMENTS
We are grateful to Mr. Hyeongjae Jeong, Department of Pathology, Gangnam Severance Hospital, for immunohistochemical stains and molecular studies that were additionally performed for the confirmation of this case.
References
1. Kesmodel SB, Terhune KP, Canter RJ, Mandel SJ, LiVolsi VA, Baloch ZW, et al. The diagnostic dilemma of follicular variant of papillary thyroid carcinoma. Surgery. 2003; 134:1005–1012.

2. Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28:1336–1340.

3. Yassin Fel-Z. Diagnostic criteria of well differentiated thyroid tumor of uncertain malignant potential; a histomorphological and immunohistochemical appraisal. J Egypt Natl Canc Inst. 2015; 27:59–67.

4. Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Cătană R, Borson-Chazot F, et al. Thyroid tumors of uncertain malignant potential: morphologic and imunohistochemical analysis of 29 cases. Pathol Res Pract. 2015; 211:320–325.

5. Noh BJ, Sung JY, Kim YW, Park YK. Recurrent thyroid papillary carcinoma in children under ten years old: report of two cases and literature review. Korean J Pathol. 2014; 48:297–301.

6. Choi SE, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. J Pathol Transl Med. 2015; 49:236–242.

7. Lin HS, Komisar A, Opher E, Blaugrund SM. Follicular variant of papillary carcinoma: the diagnostic limitations of preoperative fine-needle aspiration and intraoperative frozen section evaluation. Laryngoscope. 2000; 110:1431–1436.

8. Liu FH, Liou MJ, Hsueh C, Chao TC, Lin JD. Thyroid follicular neoplasm: analysis by fine needle aspiration cytology, frozen section, and histopathology. Diagn Cytopathol. 2010; 38:801–805.

9. Ma H, Yan J, Zhang C, Qin S, Qin L, Liu L, et al. Expression of papillary thyroid carcinoma-associated molecular markers and their significance in follicular epithelial dysplasia with papillary thyroid carcinoma-like nuclear alterations in Hashimoto’s thyroiditis. Int J Clin Exp Pathol. 2014; 7:7999–8007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download