Abstract
Although formaldehyde is well known to cause type 4 hypersensitivity, immunoglobulin E (IgE)-mediated hypersensitivity to formaldehyde is rare. Here, we report a case of recurrent generalized urticaria after endodontic treatment using a para-formaldehyde (PFA)-containing root canal sealant and present a review of previous studies describing cases of immediate hypersensitivity reactions to formaldehyde. A 50-year-old man visited our allergy clinic for recurrent generalized urticaria several hours after endodontic treatment. Prick tests to latex, lidocaine, and formaldehyde showed negative reactions. However, swelling and redness at the prick site continued for several days. The level of formaldehyde-specific IgE was high (class 4). Thus, the patient was deemed to have experienced an IgE-mediated hypersensitivity reaction caused by the PFA used in the root canal disinfectant. Accordingly, we suggest that physicians should pay attention to type I hypersensitivity reactions to root canal disinfectants, even if the symptoms occur several hours after exposure.
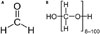
Formaldehyde is commonly used for medical applications, including disinfectants, medications, and dental procedures, especially as a root sealant.1 Formaldehyde, a low-molecular weight organic chemical, is a well-known irritant, but also an allergen acting as a hapten.2 Para-formaldehyde (PFA) is generated from formaldehyde through a condensation reaction with a typical degree of polymerization of 8–100 units (Fig. 1). PFA in powder form is converted to formaldehyde under heat upon entering the body. It is used as a fumigant, disinfectant, fungicide, and fixative. Although several case reports have described type I hypersensitivity to formaldehyde, there are no reports thereof in Korea. We report a case of recurrent generalized urticaria induced by formaldehyde through an immunoglobulin E (IgE)-mediated reaction to a root canal sealant. We also review and characterize the clinical features of formaldehyde hypersensitivity.
A 50-year-old man visited the allergy clinic because of recurrent generalized urticaria over one year. He had visited the emergency room several times for repeated generalized urticaria. The symptoms always occurred in the evening or night, beginning with axillary and inguinal pruritus, followed by erythematous conglomerated wheals over the entire body. No systemic symptoms, including wheezing, dyspnea, or hypotension, were observed. Systemic steroids and antihistamines improved the urticaria, allowing the patient to return home. His past history was notable only for cold urticaria. A thorough history revealed that the symptoms always developed at least 3–4 hours after dental treatment. However, the patient denied having erythema or pruritus on the oral mucosa and cheeks. No oral or injectable medication was administered during the dental treatment, except root canal disinfectants and local anesthesia. The root canal sealant was Depulpin® (VOCO, Cuxhaven, Germany), containing PFA and lidocaine hydrochloride. We performed prick tests and/or intradermal tests for latex (Allergopharma, Reinbek, Germany), lidocaine hydrochloride (Dai Han Pharm. Co., Seoul, Korea), and formaldehyde (Junsei Chemical Co., Tokyo, Japan) (Table 1). Physiological saline and histamine were used as controls. The skin tests showed negative immediate results. However, local swelling and erythema at the formaldehyde prick site occurred the following day and continued for several days. Laboratory tests showed an elevated serum total IgE (211.40 IU/mL), and the formaldehyde-specific IgE was 43.2 kI/L (class 4; CAP RIA Pharmacia, Uppsala, Sweden). Results were negative for 35 common allergen-specific IgE antibodies, including dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), cat, dog, cockroaches, tree, grass, weed pollen, mold, et al. (AdvanSure Allergy Screen®; LG Life Sciences, Seoul, Korea). To date, the patient has avoided formaldehyde exposure and experienced no subsequent events.
Formaldehyde is widely used in various forms, including gas, formalin solution, PFA, and trioxymethylene. Most adverse reactions are related to toxic and irritant effects, such as irritant dermatitis from cutaneous exposure and nasal, laryngeal, and bronchopulmonary symptoms from inhalation.2 Formaldehyde can also act as a sensitizer at low concentrations, inducing hypersensitivity.3 Allergic contact dermatitis to formaldehyde is the most frequent clinical manifestation.
The underlying mechanisms of formaldehyde-induced hypersensitivity have been characterized for allergic contact dermatitis. Formaldehyde acts as a contact allergen and a hapten, subsequently combining with self-proteins and inducing a hypersensitivity reaction.4 As in this case, formaldehyde can react with other molecules, such as cutaneous proteins, serum proteins, or proteins of the pulp chamber or of the periapex, and also with other elements of the root canal sealant to become a complete allergen.5
An IgE-mediated response to formaldehyde is uncommon. Antibodies against formaldehyde could develop in patients intravenously exposed to formaldehyde-sterilized dialyzers.6 Kramps, et al.7 reported that exposure to formaldehyde, even at high concentrations, rarely evokes IgE antibodies. This may explain why so few type I reactions to formaldehyde have been reported. However, recent case reports have demonstrated that type I hypersensitivity reactions are not rare, especially during dental procedures and treatments.
We summarize the cases of immediate-type reactions caused by PFA-containing dental materials in Table 25891011121314151617 including our case. Anaphylaxis, the most common manifestation, occurred in 15 cases (65%). This suggests that formaldehyde-induced type I reactions can be life-threatening. Other symptoms included general urticaria, angioedema, and local edema. Interestingly, type I hypersensitivity to formaldehyde showed time differences between exposure and reaction development. Among the reported cases, 16 patients showed symptoms within 2 to 12 hours after PFA exposure. This may be due to a gradual release of formaldehyde from water-soluble PFA. Formaldehyde in the root canal penetrates through the dentin and exits through the teeth. Formaldehyde outside the teeth is detectable 30 minutes after treatment with PFA-containing root canal fillings and gradually increases over 24 hours.18 These findings explain why hypersensitivity reactions occur several hours after dental procedures, even though the reaction is type I. In most cases, serum IgE was positive. The degree of serum IgE varied among cases, and the serum IgE level was not correlated with symptom severity.
While we did not perform patch tests, considering the pattern of progression of erythema and swelling after the prick test, this case might be viewed as a combined type I and type IV reaction. Previous reports demonstrated the coexistence of type I and type IV reactions in individual cases.6 In mice, combined type I and type IV reactions occurred when a hapten was repeatedly applied to animal skin.19 In this animal model, repeated hapten application is believed to trigger a shift in the immune response from Th1 to Th2. Type I reactions to formaldehyde are also observed in hemodialysis patients.20 This suggests that the hypersensitivity can occur regardless of exposure route. These findings highlight the importance of careful formaldehyde application, in any form, to patients with a history of formaldehyde allergy.
In conclusion, this is the first case of a type I reaction to formaldehyde present in root canal disinfectants confirmed by the presence of specific IgE in the Republic of Korea. Our findings urge clinicians to appreciate that formaldehyde allergy can cause serious clinical manifestations. Additionally, specific IgE is a useful diagnostic tool for type I hypersensitivity to formaldehyde.
Figures and Tables
Table 1
Skin Test Results

Table 2
Clinical Characteristics of 23 Patients with the Type I Allergies to (para-)formaldehyde

References
1. Rietschel RL, Fowler JF. Fisher’s contact dermatitis. 4th ed. Baltimore, MD: Williams and Wilkins;1995.
2. Johansen JD, Peter JF, Jean PL. Contact dermatitis. 5th ed. New York: Springer-Verlag Berlin Heidelberg;2011.
3. Kunisada M, Adachi A, Asano H, Horikawa T. Anaphylaxis due to formaldehyde released from root-canal disinfectant. Contact Dermatitis. 2002; 47:215–218.

4. Fehr B, Huwyler T, Wüthrich B. [Formaldehyde and paraformaldehyde allergy. Allergic reactions to formaldehyde and paraformaldehyde after tooth root treatments]. Schweiz Monatsschr Zahnmed. 1992; 102:94–97.
5. Braun JJ, Zana H, Purohit A, Valfrey J, Scherer P, Haïkel Y, et al. Anaphylactic reactions to formaldehyde in root canal sealant after endodontic treatment: four cases of anaphylactic shock and three of generalized urticaria. Allergy. 2003; 58:1210–1215.

6. Bousquet J, Rivory JP, Maurice F, Skassa-Brociek W, Larrson P, Johansson SG, et al. Allergy in chronic haemodialysis. 1. Double-blind
intravenous challenge with formaldehyde. Clin Allergy. 1987; 17:499–506.

7. Kramps JA, Peltenburg LT, Kerklaan PR, Spieksma FT, Valentijn RM, Dijkman JH. Measurement of specific IgE antibodies in individuals exposed to formaldehyde. Clin Exp Allergy. 1989; 19:509–514.

9. Ebner H, Kraft D. Formaldehyde-induced anaphylaxis after dental treatment? Contact Dermatitis. 1991; 24:307–309.
10. Wantke F, Hemmer W, Haglmüller T, Götz M, Jarisch R. Anaphylaxis after dental treatment with a formaldehyde-containing tooth-filling material. Allergy. 1995; 50:274–276.

11. el Sayed F, Seite-Bellezza D, Sans B, Bayle-Lebey P, Marguery MC, Bazex J. Contact urticaria from formaldehyde in a root-canal dental paste. Contact Dermatitis. 1995; 33:353.

12. Sayama S, Tanabe H, Kizaki J. A case of anaphylactic shock caused by dental paste for root canal. Jpn J Clin Dermatol. 1996; 50:1067–1069.
13. Wedendal PA. [Case of allergic shock in root canal therapy with tricresol formalin]. Sven Tandlak Tidskr. 1954; 47:319–321.
14. Molina C, Passemard N, Godefroid JM. [Hypersensitivity to formol and odonto-stomatology]. Rev Fr Allergol. 1971; 11:11–18.
15. Ito M, Sai M, Handa Y. Allergic reaction to formaldehyde contained in formocresol. J Dent Med. 1988; 28:897–904.
16. Gensau A, Pirkhammer D, Aberer W. Anaphylaxie durch parafomaldehydehaltige Dentalmaterialen. Allergologie. 1994; 9:439–441.
18. Wemes JC, Purdell-Lewis D, Jongebloed W, Vaalburg W. Diffusion of carbon-14-labeled formocresol and glutaraldehyde in tooth structures. Oral Surg Oral Med Oral Pathol. 1982; 54:341–346.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download