Abstract
Purpose
The purpose of this study was to introduce a method of using three-dimensional (3D) curved-multiplanar reconstruction (MPR) images for sylvian dissection during microsurgical treatment of middle cerebral artery (MCA) aneurysms.
Materials and Methods
Forty-nine patients who had undergone surgery for MCA aneurysms were enrolled. We obtained the 3D curved-MPR images along the sphenoid ridge using OsiriX MD™ imaging software, compared sylvian dissection time according to several 3D MPR image factors, and investigated the correlations between these images and intraoperative findings.
Results
Utilizing preoperative information of the sylvian fissure (SF) and peri-aneurysmal space on 3D curved-MPR images, we could predict the feasibility of sylvian dissection for a safe surgery. 3D curved-MPR images showed several features: first, perpendicular images to the sylvian surface in the same orientation as the surgeon's view; second, simultaneous visualization of the brain cortex, vessels, and cisternal space; and third, more accurate measurement of various parameters, such as depth of the MCA from the sylvian surface and the location and width of the SFs.
Sylvian dissection is a key procedure during microsurgical aneurysm clipping. Preoperative information regarding sylvian fissure (SF) anatomy is important for a safe and successful dissection. In 1984, Yasargil described four categories of SFs, based on SF shape and arachnoid characteristics, and illustrated sectional images perpendicular to the sylvian surface, which provided comprehensible information on the SF. However, common preoperative imaging studies do not produce such images.
Multiplanar reconstruction (MPR) is a method for displaying three-dimensional (3D) datasets, and allows the production of sectional images, such as original two-dimensional coronal, sagittal, and oblique images. Curved-MPR reconstructs sectional images perpendicular to a specific curved line made by the user. We made sectional images perpendicular to the sylvian surface along the sphenoid ridge line using a 3D curved-MPR technique. The purpose of this study was to introduce a method using 3D curved-MPR images for sylvian dissection in the microsurgery of middle cerebral artery (MCA) aneurysms.
We enrolled 49 patients with MCA aneurysms who underwent clipping surgery from January 2013 to December 2015. Thirty-three of the patients were female, and 16 were male. The mean age of the patients was 57.4±10.1 years (40–82 years). Forty patients had ruptured MCA aneurysms, while nine had unruptured MCA aneurysms. All of the surgeries were performed using trans-sylvian approach with a pterional craniotomy. Sylvian dissections were performed by a single surgeon with the policy of establishing proximal control of M1 before aneurysmal neck dissection.
Axial images (1.5-mm thickness) from brain computed tomography (CT) angiography were exported in a DICOM format and were imported to OsiriX MD™ imaging software (Pixmeo Inc., Geneva, Switzerland). The first step was to produce 3D MPR images by clicking "3D curved-MPR" button. The second step was to define a curved line along the sphenoid ridge on the 3D MPR images (Fig. 1). The final step involved adjusting the settings of the working screen by changing several factors, such as "Curved MPR Angle" (45–60 degree), "Thick Slab" [8–12 with maxium intensity projection (MIP) mode], "Reformation Type" (Straightened), "Resolution" (High-Res), and brightness (Fig. 2). We could then observe the serial MPR images perpendicular to the curved line by dragging the location bar on the working screen (Supplementary Video 1, only online).
The video file shows serial images perpendicular to the curved line from the anterior clinoid process (ACP) to the M2 branches distal to an aneurysm (Supplementary Video 1, only online). The frontal and temporal lobe, cisternal space, internal carotid artery, anterior cerebral artery, MCA, and the aneurysm are seen. The relationships between the anatomical structures (brain cortex, vessels, and cisternal space) are also visualized simultaneously. In the working screen, objective measurements, such as length, depth, width, and diameter, can be obtained precisely. In addition, the entire course and the depth change of the MCA in the SF were visualized (Fig. 2). These images are perpendicular to the sylvian surface and have the same left-right orientation as the surgeon's view.
Intraoperative videos were used for time measurements. We defined the sylvian dissection time as the time from the first arachnoid opening to the start of the aneurysmal neck dissection. This time was measured using the timeline of a movie player. The time spent for procedures unrelated to the sylvian dissection was excluded from our analysis.
The mean dissection time for all patients was 50±9.9 minutes. The mean dissection times were 52±10.2 minutes in the ruptured group (n=40) and 41±9.7 minutes in the unruptured group (n=9). The mean dissection time was longer in the ruptured group than in the unruptured group (p<0.05). Comparisons of dissection time using different MPR imaging parameters (location and width of the SF and depth of the MCA) were performed in the ruptured group.
The location of the SF was defined as the maximal distance from the sphenoid ridge to the SF on the MPR images (Fig. 3). The term 'herniation' has been used to describe the interdigitation of the frontal and temporal lobes.1 The SF location represents the degree of herniation, and was classified as central (<10 mm) or lateral (≥10 mm) types. The mean dissection time of the central group was shorter than that of the lateral group (p<0.05) (Fig. 3, Table 1). A long SF location, which indicates severe herniation of the frontal lobe onto the temporal lobe, was related to a long dissection time.
The width of the SF was defined as the maximal width of the cistern in the SF, was measured perpendicular to the dissection corridor (Fig. 4A), and was categorized as wide (≥3 mm), narrow (<3 mm and ≥1 mm), or adhesion (<1 mm) types. Considering the width of the SF, the patients were classified into wide (n=10), narrow (n=14), and adhesion groups (n=16) (Fig. 4, Table 1). The mean dissection time of the wide group was shorter than those of the other groups (p<0.05). In addition, the mean dissection time of the narrow group was shorter than that of the adhesion group (p<0.05).
The depth of the MCA was defined as the maximal depth of the MCA from the sylvian surface (Fig. 5), and was classified as superficial (<10 mm) or deep (≥10 mm) types. Considering the depth of the MCA, the patients were classified into the superficial group (n=18) and the deep group (n=22) (Fig. 5, Table 1). The mean dissection time of the deep group was longer than that of the superficial group (p<0.05).
During microsurgical procedures to treat ruptured MCA aneurysms, early dissection and identification of M1 are important for proximal control, which helps to reduce blood flow to the aneurysm. Although sylvian dissection is performed in the medial-to-lateral direction, the starting point of the arachnoid opening may be critical for safe dissection. By checking the distance between the ACP and the aneurysmal neck on the working screen (Fig. 6) and the surgical field (Fig. 7), we were able to establish a safety zone for the arachnoid incision. Longer distances provided wider safety zones, and shorter distances led to narrower safety zones.
3D curved-MPR images provided information on underlying cisterns and cortical adhesions at a specific point from the sylvian surface. With a wide cisternal space just below arachnoid membrane, we could readily find an underlying MCA (Fig. 8). Although we encountered a thick cortical adhesion site, a moderate to wide cisternal space, including MCA, under the adhesion was predicted (Fig. 8).
Peri-aneurysmal MPR images provided useful anatomical information on the locations of aneurysms and MCA branches in the sylvian cistern, projection, and cortical adhesion of aneurysmal dome, and were consistent with intraoperative findings (Fig. 9). Knowing the location and dome projection of an aneurysm was deemed important to a safe sylvian dissection (Fig. 10). Also, cortical adhesion of the aneurysmal dome could be detected, which should be considered during brain retraction (Fig. 11).
The SF is a gateway to aneurysms around the circle of Willis. Separation of the frontal and temporal lobes opens the subarachnoid network, unlike any other maneuver, making a SF split one of the aneurysm surgeon's most important techniques. In 1984, Yasargil described four categories of SFs based on cisternal size (large or small) and arachnoid characteristics (transparent+fragile or thickened+tough) and suggested that microsurgical dissections of the sylvian cistern during a pterional approach were increasingly more difficult as the category of cistern increases.1 In 2011, Lawton described four types of SFs: 1) atrophic fissures in older patients are wide open with minimal contact between the frontal and temporal lobes, 2) apposed fissures are more common with large areas of contact between the frontal and temporal lobes. Interdigitated fissure, either frontal-herniating, 3) or temporal-herniating, 4) are tightly apposed with contoured areas of contact, and the angle of approach must follow this rolling contour.2 In 2013, Ngando and Maslehaty3 investigated anatomic configurations of the SF and its influence on outcomes after a pterional approach for microsurgical aneurysm clipping. They described four different anatomical variants of the SF: 1) straight wide or narrow, 2) wide fissure with herniation of the frontal or temporal lobe, 3) herniation of the frontal and temporal bone or narrow, and 4) herniation of temporal and frontal lobe. They suggested that the anatomical variants of the SF may be associated with post-operative comlications, such as the formation of brain edema or ischemic lesions.3
Pre-operative information regarding SF anatomy, nearby vessels, and aneurysms is of utmost importance in sylvian dissection and is required for safe microsurgical treatment. However, conventional studies, such as brain CT, CT angiography, MRI, and cerebral angiography, have limitations in discriminating a patient's SF category or type among the above-described categories or types, preoperatively. 3D curved-MPR images could provide useful information for discriminating a SF category or type, deciding surgical approach, and direction of sylvian dissection. The location and width of the SF and the depth of the MCA could be measured at any point of the SF. The entire course of the MCA in the SF, and especially its depth changes, and surface venous structures could be visualized. Because accurate measurement of various anatomical factors is possible, quantification and classification of these factors could be done. Also, peri-aneurysmal information, such as the locations and projections of MCA aneurysms, and the locations of M2 branches could be obtained. All of the above information was easily comprehensible, because the images produced were perpendicular to the sylvian surface and had the same right-left orientation as that of the surgeon's view. The images revealed anatomical structures, such as the cortex, blood vessels, and cisterns, simultaneously. Finally, based on preoperative information of the SF and peri-aneurysmal space with 3D curved-MPR images and conventional imaging studies, we could predict the feasibility of sylvian dissection for safe surgeries. Additionally, we suggest that the utilization of 3D curved-MPR image in sylvian dissection may be useful in training young neurosurgeons.
A surgical approach is determined based on the surgeon's preference and various anatomical factors.4 Traditionally, aneurysms in the anterior circulation have been addressed by frontotemporal craniotomy using the pterional approach.1 Perneczky, et al.5 introduced the keyhole concept in neurosurgery. Since the keyhole technique is less-invasive and requires small skin incisions and craniotomies and leads to minimal brain exposure, many neurosurgeons have reported the usefulness of modified pterional keyhole approaches.67891011 Hernesniemi, et al.7 suggested the use of focused sylvian opening, which is a less-invasive alternative to the classical wide sylvian opening, which is used for the microsurgical management of most MCA aneurysms. 12 However, these less-invasive approaches require extensive experience with the conventional pterional approach and with pre-operative evaluation of the feasibility of sylvian dissection and clipping. Huh4 suggested, based on published studies of surgical approaches for sylvian dissection, that it is mandatory to evaluate the depth of the SF and the lengths and courses of M1 and M2, as well as the projections and sizes of MCA bifurcation aneurysms, for successful neck clipping.131415
There are some limitations to this study. First, there was some degree of image distortion, which is due to the process of straightening the curved line made along the sphenoid ridge. Second, removal of cerebrospinal fluid using extraventricular drainage or the opening of the cisterns can cause changes in the cisternal anatomy. Third, this technique provides limited information on sylvian veins, such as the relationship between sylvian veins and the aneurysm dome. To date, it is impossible to show sylvian vein orientation.
In future studies, we aim to include MPR image production with thinner axial images (0.5 mm) and to develop an algorithm for the planning of sylvian dissection. In addition to conventional image studies, 3D curved-MPR images seem to provide useful information for sylvian dissection in the microsurgery of MCA aneurysms.
Figures and Tables
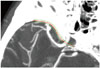 | Fig. 1Curved line along the sphenoid ridge. The line was produced by placing several points along the sphenoid ridge on 3D MPR images. 3D, three-dimensional; MPR, multiplanar reconstruction. |
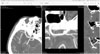 | Fig. 2The working screen. The left panel shows a right MCA aneurysm on the 3D MPR image. The middle panel shows a straightened image of the curved line on the sphenoid ridge, and the right three panels display images perpendicular to the straightened line at three points. MCA, middle cerebral artery; 3D, three-dimensional; MPR, multiplanar reconstruction. |
 | Fig. 3Location of the sylvian fissure. (A) Central group (<10 mm). (B) Lateral group (≥10 mm). The location of the sylvian fissure (white arrow) is the distance between the anterior clinoid process and the sylvian fissure on the surface. A lateral location of the sylvian fissure means that there is a herniation of the frontal lobe onto the temporal lobe. |
 | Fig. 4Width of the sylvian fissure. (A) Wide group (≥3 mm). (B) Narrow group (<3 mm and ≥1 mm). (C) Adhesion group (<1 mm). White arrow is the measurement of the width of the sylvian fissure, which is perpendicular to the direction of dissection corridor (black arrow). |
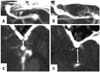 | Fig. 5Depth of the MCA. (A and C) Superficial group [maximal depth (arrow) of the MCA < 10 mm]. (B and D) Deep group [maximal depth (arrow) ≥10 mm]. MCA, middle cerebral artery. |
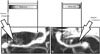 | Fig. 6Safety zone for the arachnoid incision. (A) Long safety zone. (B) Short safety zone. Large arrows reveal the oblique direction of the sylvian dissection. Gradations in the rectangles marked as "safety zone" indicate the degree of risk. |
 | Fig. 7Intra-operative measurements. Measuring the distance from the anterior clinoid process with a paper ruler. |
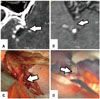 | Fig. 8Cisternal space under the sylvian surface. (A and C) Wide cisternal space (arrows) just under the surface arachnoid membrane. (B and D) Middle cerebral artery and moderate cisternal space under thick cortical adhesion (arrows). This an MR MPR image. MPR, multiplanar reconstruction. |
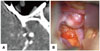 | Fig. 9Location of an aneurysm and MCA branches in the sylvian cistern. (A) MPR image. (B) Intraoperative capture image. Two images provide the following anatomical information: a moderate sylvian cistern, the shallow location of the superior branch, the aneurysm under the temporal lobe, and deep location of the inferior branch. MCA, middle cerebral artery; MPR, multiplanar reconstruction. |
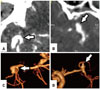 | Fig. 10Projection of aneurysmal dome. (A and C) The direction of the aneurysmal dome is opposite to the surface. During sylvian dissection, the secure of aneurysmal neck and branches (arrows) is possible. (B and D) The direction is to sylvian surface, and the dome is located just below superficial sylvian veins on the temporal lobe (arrows). |
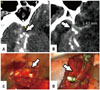 | Fig. 11Cortical adhesion of aneurysmal dome. (A and C) Aneurysmal dome (arrows) adheres to the frontal lobe. (B and D) Aneurysmal dome (arrows) contacts with the temporal lobe. Temporary clips for safe neck dissection are shown at the proximal and distal site of the aneurysm. |
Table 1
Mean Dissection Times in Patients with Ruptured MCA Aneurysms

ACKNOWLEDGEMENTS
The Ministry of Trade, Industry & Energy (MI, Korea) provided financial support in the form of the Technology Innovation Program [Advanced Technology Center (ATC), 10048523, Development of a fluorography system with a 50% lower irradiation dose and image quality on par with angiographic equipment to global leaders of the next generation].
References
1. Yaşargil MG. Microneurosurgery. New york: Thieme-Stratton;1984.
2. Lawton MT. Seven Aneurysms: Tenets and Techniques for Clipping. New York: Thieme;2011.
3. Ngando HM, Maslehaty H, Schreiber L, Blaeser K, Scholz M, Petridis AK. Anatomical configuration of the Sylvian fissure and its influence on outcome after pterional approach for microsurgical aneurysm clipping. Surg Neurol Int. 2013; 4:129.

4. Huh SK. [Microsurgical anatomy of the middle cerebral artery]. J Korean Neurosurg Soc. 1998; 27:1769–1773.
5. Perneczky A, van Lindert E, Müller-Forell W, Fries G. Keyhole concept in neurosurgery. New York: Thieme;1999.
6. Harland SP, Hussein A, Gullan RW. Modification of the standard pterional approach for aneurysms of the anterior circle of Willis. Br J Neurosurg. 1996; 10:149–153.

7. Hernesniemi J, Ishii K, Niemelä M, Smrcka M, Kivipelto L, Fujiki M, et al. Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl. 2005; 94:17–21.

8. Lan Q, Gong Z, Kang D, Zhang H, Qian Z, Chen J, et al. Microsurgical experience with keyhole operations on intracranial aneurysms. Surg Neurol. 2006; 66:Suppl 1. S2–S9.

9. Mitchell P, Vindlacheruvu RR, Mahmood K, Ashpole RD, Grivas A, Mendelow AD. Supraorbital eyebrow minicraniotomy for anterior circulation aneurysms. Surg Neurol. 2005; 63:47–51.

10. Mori K, Osada H, Yamamoto T, Nakao Y, Maeda M. Pterional keyhole approach to middle cerebral artery aneurysms through an outer canthal skin incision. Minim Invasive Neurosurg. 2007; 50:195–201.

11. Yamahata H, Tokimura H, Tajitsu K, Tsuchiya M, Taniguchi A, Hirabaru M, et al. Efficacy and safety of the pterional keyhole approach for the treatment of anterior circulation aneurysms. Neurosurg Rev. 2014; 37:629–636.

12. Elsharkawy A, Niemelä M, Lehecčka M, Lehto H, Jahromi BR, Goehre F, et al. Focused opening of the sylvian fissure for microsurgical management of MCA aneurysms. Acta Neurochir (Wien). 2014; 156:17–25.

13. Ogilvy CS, Crowell RM, Heros RC. Surgical management of middle cerebral artery aneurysms: experience with transsylvian and superior temporal gyrus approaches. Surg Neurol. 1995; 43:15–22.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download