Abstract
Purpose
The increased tolerability of enteric-coated mycophenolate sodium (EC-MPS), compared to mycophenolate mofetil, among kidney transplant recipients has the potential to facilitate cyclosporine (CsA) minimization. Therefore, a prospective trial to determine the optimum EC-MPS dose in CsA-based immunosuppression regimens is necessary.
Materials and Methods
A comparative, parallel, randomized, open-label study was performed for 140 patients from four centers to compare the efficacy and tolerability of low dose CsA with standard dose EC-MPS (the investigational group) versus standard dose CsA with low dose EC-MPS (the control group) for six months in de novo kidney transplant recipients. Graft function, the incidence of efficacy failure [biopsy-confirmed acute rejection (BCAR), death, graft loss, loss to follow-up], and adverse events were compared.
Results
The mean estimated glomerular filtration rate (eGFR) of the investigational group at six months post-transplantation was non-inferior to that of the control group (confidence interval between 57.3 mL/min/1.73m2 and 67.4 mL/min/1.73 m2, p<0.001). One graft loss was reported in the control group, and no patient deaths were reported in either group. The incidence of BCAR of the investigational group was 8.7%, compared to 18.8% in the control group (p=0.137), during the study period. There were no significant differences (p>0.05) in the incidence of discontinuations and serious adverse events (SAE) between the groups.
Since calcineurin inhibitors (CNIs), such as cyclosporine (CsA) and tacrolimus, were introduced in kidney transplantation (KT), the incidence of acute rejection has been reduced, and the graft survival rate early after transplantation has been improved. However, despite dramatic reductions in acute rejection rates over time, long-term graft survival has not improved an appreciable extent.12 Thus, redirecting attention from early endpoints toward the process of long-term graft loss may be necessary.3 Many contributory factors for the lack of improvement in late graft survival have been postulated,1 and among the causes of later graft failure, the leading contributor to renal dysfunction and eventual graft loss is chronic allograft injury (CAI). Seemingly, prevention of and intervention in CAI appear to be appropriate strategies for overcoming late renal graft failure. Meanwhile, pathologic changes in CNI nephrotoxicity are almost universal at ten years, and can exacerbate CAI.4 To alleviate CNI nephrotoxicity, reduction and/or withdrawal of CNI may be necessary.
To reduce the risk of nephrotoxicity, regimens with reduced CNI exposure may present a reasonable approach. Entericcoated mycophenolate sodium (EC-MPS) (Myfortic®, Novartis Pharma AG, Basel, Switzerland) is a formulation of mycophenolic acid that delivers the same efficacy benefits as mycophenolate mofetil (MMF), with the additional potential to reduce gastro-intestinal symptom burden.56 EC-MPS and MMF have been found to provide similar pharmacodynamic effects for inosine monophosphate dehydrogenase enzyme activity, and are therapeutically equivalent in de novo and maintenance kidney transplant recipients.78 The better gastro-intestinal tolerability of EC-MPS in comparison with MMF in maintenance patients has the potential to facilitate newer strategies, including CNI minimization. To reduce CsA exposure, the appropriate dosage of EC-MPS should be designed to achieve the therapeutic goal of immunosuppression in the early period after KT.9
According to the MORE Registry study,10 68.1% of 904 de novo kidney recipients received EC-MPS rather than MMF as a part of their immunosuppressive regimen at the time of hospital discharge, undertaken at 40 transplant centers in the United States. In addition, despite more than 50% of patients receiving less than the maximum recommended dose of these drugs by six months post-transplantation, their outcomes were excellent. This suggests that a prospective trial to investigate the optimum EC-MPS dose in CNI-based immunosuppression regimens is warranted.
Due to the relative scarcity of previous reports on the CsA-sparing effect of standard dose EC-MPS, this study was designed to compare the efficacy and tolerability of reduced-dose CsA with standard-dose EC-MPS versus standard-dose CsA with reduced-dose EC-MPS, combined with basiliximab and corticosteroids, in de novo kidney recipients.
This study was designed as a prospective, multicenter (four transplant centers in Korea), randomized, controlled, parallel-group trial in recipients (aged 20–65) of de novo KT. The key exclusion criteria included recipients of multiple organ transplants or organs donated after cardiac death; donors either younger than 15 or older than 65 years; recipients of ABO-incompatible transplants; recipients with antibodies against the human leukocyte antigens of the donor organ; recipients' with leukocyte counts of less than 2500 per µL, neutrophils less than 1500 per µL, or platelets less than 75000 per µL; and those with evidence of severe liver disease.
The study was conducted in compliance with Good Clinical Practice guidelines. The study protocol was approved by the independent Institutional Review Boards of each center, and the procedures followed in the trial were in accordance with the Declaration of Helsinki. This study was registered with ClinicalTrials.gov (registration identifier=NCT01817322).
The patients who were enrolled in the study after providing written informed consent received induction treatment with basiliximab (Simulect®, Novartis, Basel, Switzerland), CsA (Sandimmun Neoral®, Novartis, Basel, Switzerland), EC-MPS (Myfortic®, Novartis), and corticosteroids. Basiliximab was given just prior to transplantation and four days after transplantation. CsA was given orally at a starting dose of 10 mg/kg/day from one day before transplantation. According to a previous study,11 methyl-prednisolone was injected intravenously at the following doses: 500 mg on the day of operation, 250 mg on the day after, and corticosteroids were tapered to a maintenance dose of more than 5 mg a day (prednisolone or equivalent).
For the investigational group, according to previous studies on CsA minimization,1213 the CsA dose was individually adjusted with a target trough blood level between 100 ng/mL and 200 ng/mL within a month after transplantation. The target blood trough levels of CsA were reduced to between 75 ng/mL and 150 ng/mL until two months post-transplantation, and were further reduced to between 50 ng/mL and 125 ng/mL until four months, and then to between 50 ng/mL and 100 ng/mL until six months post-transplantation. The target dose of EC-MPS was 1440 mg/day, orally, for the investigational group throughout the follow-up period.
For the control group, the CsA dose was individually adjusted with a goal trough blood level between 200 ng/mL and 300 ng/mL within a month after transplantation. The target blood trough levels of CsA were reduced to between 150 ng/mL and 250 ng/mL until two months post-transplantation, further reduced to between 125 ng/mL and 200 ng/mL until four months, and then to between 100 ng/mL and 200 ng/mL until six months post-transplantation. The target dose of EC-MPS was 720 mg/day, orally, throughout the follow-up period.
Study visits took place on the day before transplantation (baseline) and at one month, two months, four months, and six months post-transplantation. At each visit, a complete physical examination was performed, and laboratory values concerning the kidneys, liver, hematology, proteinuria, and trough levels of CsA were measured. Blood pressure, weight, and any problems between visits were documented. We examined renal function with serum creatinine level and with estimated glomerular filtration rates (eGFR) according to the Modification of Diet in Renal Disease (MDRD) formula.14 Data were recorded, entered into an electronic database, and re-evaluated by external monitors. Study monitoring and database analyses were performed according to Good Clinical Practice guidelines, and all adverse events (AEs) and serious adverse events (SAEs) were documented.
The primary efficacy endpoint was renal graft function, which was assessed with eGFR by the MDRD formula, at six months post-transplantation. Other prospectively defined endpoints included a composite variable of the incidence of efficacy failure that included biopsy-confirmed acute rejection (BCAR), graft loss, death, or loss to follow-up until six months post-transplantation. Patients with clinical findings suggestive of acute rejection underwent biopsies before initiation or within 48 hours of initiation of anti-rejection therapy, and biopsy specimens were graded according to Banff criteria.15 Rejection was treated with corticosteroids, either with or without anti-thymocyte globulin, depending on the histological grade and clinical course. Allograft loss was presumed to have occurred if a patient began dialysis and could not subsequently be removed from dialysis. Safety assessments included incidences of AEs and SAEs. According to a previous study,11 AE was defined as any untoward medical occurrence, including exacerbation of a pre-existing condition, in a patient in a clinical investigation who has received a pharmaceutical product. The event did not necessarily have a causal relationship with this treatment. SAE was defined as any AE with undesirable signs, symptoms, or medical conditions that met any one of the following criteria: 1) was fatal or life-threatening, 2) resulted in persistent or significant disability/incapacity, 3) required hospitalization or the prolongation of existing hospitalization, 4) was a congenital anomaly/birth defect, or 5) was an important medical event that might deteriorate the patient and require medical or surgical intervention to prevent one of the other outcomes listed above. Serial laboratory results and the proportion of patients with clinically notable abnormalities were reported.
A sample size of 70 for each treatment group was determined for the primary endpoint by assuming a significance level (alpha) of 0.025, 90% power, with a non-inferiority margin [margin of equivalence of 7.5 mL/min and standard deviation (SD) of 25.1 mL/min] with reference to a previous study16 and a 10% dropout rate.
Eligible individuals were randomly assigned in a 1:1 ratio to either the investigational group (low dose CsA+standard dose EC-MPS) or the control group (standard dose CsA+low dose EC-MPS). Randomization assignments were centrally released via an electronic case report form prior to transplantation. For the randomization of enrolled subjects, a random seed with a stratification factor for each research institution was generated. Block and block size were randomly assigned, and both the enrolled subjects and care providers involved in this study were blinded until randomization.
Categorical variables were analyzed using chi-square testing with SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA). All categorical values were expressed as a percentage of the group from which they were derived, and their p-values were calculated using Fisher's exact test. Continuous variables were analyzed using t-test, and expressed as a mean±SD. In this study, p values <0.05 were considered significant.
Patients were enrolled from July 2011 to February 2013. Fig. 1 shows the profile of this clinical trial. Of 140 randomized patients, 138 (69 in the investigational group and 69 in the control group) were provided with at least one dose of the study drug after transplantation, and comprised the intention-to-treat (ITT) population. Ninety-nine (49 in the investigational group and 50 in the control group) patients completed the study follow-up and completed the study drug; they comprised the per-protocol (PP) population. The main reasons for discontinuation before the end of the study at six months post-transplant were protocol violation, rejection, unsatisfactory therapeutic effects, and loss at follow-up (Fig. 1). The characteristics of the patients and their donors were similar between the groups, as displayed in Table 1.
The mean blood trough levels and doses of CsA at one, two, four, and six months post-transplantation are shown in Table 2. The mean blood trough levels of CsA in the investigational group at one, two, four, and six months post-transplantation were 178.0±69.3, 146.0±56.5, 115.3±46.7, and 103.5±38.9 ng/mL, respectively. Those in the control group were 221.2±68.8, 189.4±78.4, 144.9±43.0, and 142.9±44.8 ng/mL, respectively. Reflecting lower CsA exposure, CsA trough levels at one, two, four, and six months were significantly lower (p<0.05) in the investigational group than in the control group.
Overall compliance with the study drug (EC-MPS) was 96.0% in the investigational group and 97.7% in the control group.
In the ITT population, the mean eGFR at one month post-transplantation was 64.6±21.0 mL/min/1.73 m2 in the investigational group, compared to 58.7±18.2 mL/min/1.73 m2 in the control group (p=0.091). At two months post-transplantation, it was 62.2±15.9 mL/min/1.73 m2 in the investigational group, compared to 57.6±15.2 mL/min/1.73 m2 in the control group (p=0.118), and at four months post-transplantation, it was 61.2±14.7 mL/min/1.73 m2 in the investigational group, compared to 57.5±13.2 mL/min/1.73 m2 in the control group (p=0.173). At six months post-transplantation, it was 62.3±17.3 mL/min/1.73 m2 in the investigational group, compared to 58.6±14.4 mL/min/1.73 m2 in the control group (p=0.237), as displayed in Fig. 2. By the non-inferiority test, the mean eGFR of the investigational group at six months post-transplantation was significantly non-inferior to that of the control group (the confidence interval was between 57.5 mL/min/1.73 m2 and 67.2 mL/min/1.73 m2, p<0.001).
In the PP population, the mean eGFR at one month post-transplantation was 65.7±21.1 mL/min/1.73 m2 in the investigational group, compared to 60.0±17.5 mL/min/1.73 m2 in the control group (p=0.148). At two months post-transplantation, it was 62.8±16.6 mL/min/1.73 m2 in the investigational group, compared to 58.5±15.2 mL/min/1.73 m2 in the control group (p=0.176), and at four months post-transplantation, it was 61.8±15.2 mL/min/1.73 m2 in the investigational group, compared to 57.3±13.3 mL/min/1.73 m2 in the control group (p=0.123). Finally, at six months post-transplantation, it was 62.3±17.7 mL/min/1.73 m2 in the investigational group, compared to 58.6±14.4 mL/min/1.73 m2 in the control group (p=0.246), as displayed in Fig. 2. By the non-inferiority test, the mean eGFR of the investigational group at six months post-transplantation was significantly non-inferior to that of the control group (confidence interval: 57.3 mL/min/1.73 m2 and 67.4 mL/min/1.73 m2, p<0.001).
In the ITT population, the overall incidence of treated BCAR within six months post-transplantation in the investigational group was 8.7%, compared to 18.8% in the control group (p=0.137). One graft loss in the control group and no patient deaths in either group were reported.
Among the 138 patients in the ITT population, 122 (88.4%) experienced an AE. Sixty-two (89.9%) of the 69 patients in the investigational group and 60 (87.04%) of the 69 patients in the control group reported an AE during the study period. The incidence of AE was not significant between the groups (p=0.431), and the incidence of SAEs, severe AE, and AE leading to study drug discontinuation were not statistically significant (p=0.377, 0.699, and 0.247, respectively). A total of 366 AEs were reported: 177 in the investigational group and 189 in the control group. The most frequently reported AEs by system organ class were infections (22.0% in the investigation group and 17.5% in the control group, p=0.343), gastrointestinal disorders (13.0% in the investigation group and 16.4% in the control group, p=0.456), and metabolism and nutrition disorders (9.0% in the investigation group and 9.0% in the control group, p=0.809). The incidence of AEs by system organ class was generally similar between the groups and the majority of AEs were mild-to-moderate in severity (Table 3).
Laboratory values, including white blood cell count, hemoglobin, platelet count, cholesterol, and transaminase profiles, at one month and six months post-transplantation were comparable between the groups (Table 4).
Our experience with using standard dose EC-MPS in combination with reduced-exposure CsA and steroids in de novo KT provides additional strong evidence that this strategy is safe and efficacious. The allograft function of the investigational group (low dose CsA with standard dose EC-MPS) was non-inferior to that of the control group (standard dose CsA with low dose EC-MPS). This result suggests that standard dose EC-MPS can elicit CNI minimization and potentially reduce CNI nephrotoxicity, preserving renal function. Given the immunosuppression protocol, fewer side effects that are usually associated with the standard dose EC-MPS were reported. In terms of the incidence of acute rejection, standard dose EC-MPS combined with reduced-exposure CsA revealed as low an incidence of acute rejection as that of standard dose CsA with low dose EC-MPS. According to this result, the standard dose of EC-MPS combined with low-dose CsA can be a powerful and efficacious combination for preventing acute rejection. A previous study also reported that reduced CsA dose (target trough levels of 50–100 ng/mL) with MMF was efficient at preventing acute rejection and preserving kidney function in de novo KT.12 Another previous randomized trial reported that there was significant improvement in eGFR in the low-exposure CsA group, compared to the standard-exposure group.17
Several strategies exist to spare CNIs, including the use of agents, such as MMF, EC-MPS, sirolimus, everolimus, or belatacept. Mycophenolic acid derivatives have been used successfully to facilitate CNI minimization, improving short-term renal function after KT. Although MMF is known to be therapeutically equivalent to EC-MPS in de novo renal transplantation,18 gastrointestinal symptoms have been more frequently reported for MMF.819 A previous study reported that histologic changes in MMF-related enterocolitis included apoptosis, dilated crypts lined by attenuated epithelial cells, crypt loss, clusters of residual endocrine cells, and edematous lamina propria with sparse inflammatory cells.20 In the literature, clinical studies reporting on the CsA-sparing effect of EC-MPS are relatively fewer than those of MMF.
A recent study demonstrated that an intensified dose (2160 mg/day) of EC-MPS facilitated CsA sparing early after KT.21 However, AEs with a suspected relationship to the study drug were reported in 69.8% and 50.8% of patients in the intensified and standard regimen groups, respectively (p=0.032), in another report.22 Similar to a recent report,23 we investigated the CsA-sparing effect of standard dose EC-MPS in de novo kidney transplant patients. Unlike the aforementioned study, our study results suggested that low dose CsA with standard dose EC-MPS versus standard dose CsA with low dose EC-MPS is therapeutically equivalent in de novo renal transplant recipients.
To minimize or avoid CNI nephrotoxicity, the minimization or total avoidance of CNI exposure should be emphasized. The role of EC-MPS has been examined in protocols minimizing CsA.2425 In these studies, investigations into the use of EC-MPS with both standard- and low-dose CsA have demonstrated excellent efficacy in both de novo and maintenance patients. Given that most transplant centers, even in Korea, prefer to reduce the dose of EC-MPS with tacrolimus,10 our results provide strong evidence that reducing CsA with standard-dose EC-MPS could be therapeutically equivalent and preferable to reducing EC-MPS with a standard dose of CsA, not only in terms of efficacy but also for the safety of these immunosuppressive regimens.
Our study has several limitations. First are the relatively short follow-up time and small study population. Second, we only showed the non-inferiority of the study group (low-dose CsA with standard dose EC-MPS). Although, previous studies have outlined the efficacy and safety of low-dose CsA,1217 recently, tacrolimus has been primarily used for CNI in KT.11 Therefore, we believe further studies focusing on optimal CNI-sparing regimes with MMF in de novo KT are needed.
In conclusion, the results of this study provide evidence that 1) CsA minimization using standard-dose EC-MPS keeps the incidence of acute rejection and additional risks as low as conventional immunosuppression and that 2) this facilitates the minimization of CsA exposure and provides therapeutic equivalences in terms of the incidence of acute rejection, renal graft function, and safety issues.
Figures and Tables
Fig. 2
Graft renal function measured by estimated glomerular filtration rates (Modification of Diet in Renal Disease) (ITT and PP population). ITT, intention-to-treat; PP, per-protocol; CsA, cyclosporine; EC-MPS, enteric-coated mycophenolate sodium.

Table 1
Baseline Demographics and Clinical Characteristics (ITT Population)*

ITT, intention-to-treat; CsA, cyclosporine; EC-MPS, enteric-coated mycophenolate sodium; CAPD, continuous ambulatory peritoneal dialysis; HLA, human leukocyte antigen.
*Continuous variables are expressed as the mean±standard deviation and their p-values are calculated with t-test. Categorical variables are expressed as number (%) and their p-values are calculated with Fisher's exact test.
Table 2
The Blood Trough Level and Dose of CsA (PP Population)*
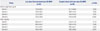
Table 3
AEs Over 6 Months of Treatment (ITT Population)*

Table 4
Laboratory Values at 1 Month and 6 Months Post-Transplantation (ITT Population)*

ACKNOWLEDGEMENTS
This work was financially provided by Novartis Pharmaceuticals AG (Basel, Switzerland).
The authors thank the Clinical Trial Center at Ajou University Hospital for their support.
References
1. Tantravahi J, Womer KL, Kaplan B. Why hasn't eliminating acute rejection improved graft survival? Annu Rev Med. 2007; 58:369–385.

2. Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004; 4:378–383.

3. Meier-Kriesche HU, Schold JD, Kaplan B. Long-term renal allograft survival: have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant. 2004; 4:1289–1295.

4. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004; 78:557–565.

5. Bolin P, Tanriover B, Zibari GB, Lynn ML, Pirsch JD, Chan L, et al. Improvement in 3-month patient-reported gastrointestinal symptoms after conversion from mycophenolate mofetil to entericcoated mycophenolate sodium in renal transplant patients. Transplantation. 2007; 84:1443–1451.

6. Dalal P, Grafals M, Chhabra D, Gallon L. Mycophenolate mofetil: safety and efficacy in the prophylaxis of acute kidney transplantation rejection. Ther Clin Risk Manag. 2009; 5:139–149.
7. Budde K, Glander P, Krämer BK, Fischer W, Hoffmann U, Bauer S, et al. Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in maintenance renal transplant recipients receiving tacrolimus: clinical, pharmacokinetic, and pharmacodynamic outcomes. Transplantation. 2007; 83:417–424.

8. Salvadori M, Bertoni E, Budde K, Holzer H, Civati G, Lien B, et al. Superior efficacy of enteric-coated mycophenolate vs mycophenolate mofetil in de novo transplant recipients: pooled analysis. Transplant Proc. 2010; 42:1325–1328.

9. Cooper M, Salvadori M, Budde K, Oppenheimer F, Sollinger H, Zeier M, et al. Enteric-coated mycophenolate sodium immunosuppression in renal transplant patients: efficacy and dosing. Transplant Rev (Orlando). 2012; 26:233–240.

10. Langone A, Shihab F, Pankewycz O, Doria C, Wiland A, McCague K, et al. Long-term dosing patterns of enteric-coated mycophenolate sodium or mycophenolate mofetil with tacrolimus after renal transplantation. Clin Transplant. 2014; 28:961–967.

11. Oh CK, Huh KH, Lee JS, Cho HR, Kim YS. Safety and efficacy of conversion from twice-daily tacrolimus to once-daily tacrolimus one month after transplantation: randomized controlled trial in adult renal transplantation. Yonsei Med J. 2014; 55:1341–1347.

12. Ekberg H, Grinyó J, Nashan B, Vanrenterghem Y, Vincenti F, Voulgari A, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant. 2007; 7:560–570.

13. Hernández D, Miquel R, Porrini E, Fernández A, González-Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation. 2007; 84:706–714.

14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–470.

15. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–723.

16. Ekberg H, Tedesco-Silva H, Demirbas A, Vütko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007; 357:2562–2575.

17. Etienne I, Toupance O, Bénichou J, Thierry A, Al Najjar A, Hurault de Ligny B, et al. A 50% reduction in cyclosporine exposure in stable renal transplant recipients: renal function benefits. Nephrol Dial Transplant. 2010; 25:3096–3106.

18. Sollinger H. Enteric-coated mycophenolate sodium: therapeutic equivalence to mycophenolate mofetil in de novo renal transplant patients. Transplant Proc. 2004; 36:2 Suppl. 517S–520S.

19. Ortega F, Sánchez-Fructuoso A, Cruzado JM, Gómez-Alamillo JC, Alarcón A, Pallardó L, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation. 2011; 92:426–432.

20. Panarelli NC. Drug-induced injury in the gastrointestinal tract. Semin Diagn Pathol. 2014; 31:165–175.

21. Cai L, Zeng F, Liu B, Wei L, Chen Z, Jiang J. A single-centre, open-label, prospective study of an initially short-term intensified dosing regimen of enteric-coated mycophenolate sodium with reduced cyclosporine A exposure in Chinese live-donor kidney transplant recipients. Int J Clin Pract Suppl. 2014; (181):23–30.

22. Arns W, Sommerer C, Glander P, Ariatabar T, Porstner M, May C, et al. A randomized trial of intensified vs. standard dosing for enteric-coated mycophenolate sodium in de novo kidney transplant recipients: results at 1 year. Clin Nephrol. 2013; 79:421–431.

23. Chadban S, Eris J, Russ G, Campbell S, Chapman J, Pussell B, et al. Enteric-coated mycophenolate sodium in combination with full dose or reduced dose cyclosporine, basiliximab and corticosteroids in Australian de novo kidney transplant patients. Nephrology (Carlton). 2013; 18:63–70.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download