Abstract
Purpose
Angiopoietin-1 (Ang1) is a critical factor for vascular stabilization and endothelial survival via inhibition of endothelial permeability and leukocyte- endothelium interactions. Hence, we hypothesized that treatment with umbilical cord mesenchymal stem cells (UCMSCs) carrying the Ang1 gene (UCMSCs-Ang1) might be a potential approach for acute lung injury (ALI) induced by lipopolysaccharide (LPS).
Materials and Methods
UCMSCs with or without transfection with the human Ang1 gene were delivered intravenously into rats one hour after intra-abdominal instillation of LPS to induce ALI. After the rats were sacrificed at 6 hours, 24 hours, 48 hours, 8 days, and 15 days post-injection of LPS, the serum, the lung tissues, and bronchoalveolar lavage fluid (BALF) were harvested for analysis, respectively.
Results
Administration of fluorescence microscope confirmed the increased presence of UCMSCs in the injured lungs. The evaluation of UCMSCs and UCMSCs-Ang1 actions revealed that Ang1 overexpression further decreased the levels of the pro-inflammatory cytokines TNF-α, TGF-β1, and IL-6 and increased the expression of the anti-inflammatory cytokine IL-10 in the injured lungs. This synergy caused a substantial decrease in lung airspace inflammation and vascular leakage, characterized by significant reductions in wet/dry ratio, differential neutrophil counts, myeloperoxidase activity, and BALF. The rats treated by UCMSCs-Ang1 showed improved survival and lower ALI scores.
Acute lung injury (ALI) is the major cause of mortality in critically ill children, characterized by an acute severe inflammatory response in neutrophilic alveolitis,1 with pulmonary edema due to alveolar epithelial-interstitial-endothelial injury. Lipopolysaccharide (LPS), a microbial pathogen, is one of the most important components of the cell wall of Gram-negative bacteria and plays a key role in the pathogenesis of ALI.23 Current treatments of ALI induced by LPS remain ineffective, and ALI-associated mortality still remains higher.4
Mesenchymal stem cells (MSCs) isolated from bone marrow are multipotent, self-renewing cells that can differentiate into different tissues, such as bone, fat, fibroblasts, cartilage, and muscle, and have been found to have beneficial effects in some diseases, including traumatic brain injury.5 Mei, et al.1 infused MSCs intravenously thirty minutes after intra-tracheal LPS and reported a significant reduction in bronchoalveolar lavage fluid (BALF) total cell and neutrophil counts three days later in several lung diseases. Additionally, decreases in inflammatory infiltrates, interstitial edema, and interalveolar septal thickening were confirmed by histologic analysis. However, because of early strong inflammatory response, a large number of inflammatory cells were noted, and inflammatory medium and inflammatory factors for the survival of the transplanted cells were unfavorable, which might be one of the reasons for limited repairment capatity of MSCs.6
Angiopoietin-1 (Ang1) can significantly promote neovascularization via the inhibition of leukocyte-endothelium interactions and endothelial permeability,2 and during this course, vascular endothelial growth factor (VEGF) might be released from genetically modified adipose stem cells.7 Xu, et al.8 reported that LPS-induced lung injury was alleviated in mice treated with BM-MSCs (from bone marrow, BM) carrying Ang1 (MSCs-Ang1), compared with mice treated with only MSCs or Ang1 in vivo. Therein, the expression of Ang1 protein increased in the MSCs-Ang1 recipient lungs, and cells originating from MSCs could be detected in the recipient lung tissues for about two weeks after MSCs injection.
Meanwhile, however, studies have yet to suggest that umbilical cord-derived mesenchy mal stem cells (UCMSCs) with or without Ang1 (UCMSCs-Ang1) is beneficial or not in ALI, and its potential effect in improving LPS-induced ALI has not been evaluated. In light of previous studies, we assumed that UCMSCs carrying Ang1 (UCMSCs-Ang1) could result in a further improvement in both systemic inflammation and alveolar permeability and UCMSCs-Ang1 gene therapy might be a potential novel strategy for treatment of ALI.
Umbilical cords (n=10, normal pregnancies, approved by the human research Ethics Committee of the Qilu Hospital, Shandong University) were excised and washed in a 0.1 mol/L phosphate buffer (pH 7.4) to remove excess blood. The cords were dissected and the blood vessels were removed. The remaining tissue was cut into small pieces (1–2 mm3) and placed in plates with low-glucose Dulbecco-modified Eagle medium (L-DMEM) (Gibco-BRL, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 2 ng/mL of epidermal growth factor (R&D Systems, Minneapolis, MN, USA), 2 ng/mL of fibroblast growth factor (R&D Systems), 2 ng/mL of (VEGF; R&D Systems), and 100 µg/mL of streptomycin (Gibco-BRL), 100 U/mL penicillin. Cultures were maintained with 5% CO2 at 37℃ in a humidified atmosphere. The media were changed every 3–4 days. Adherent cells proliferated from individual explanted tissues 7–12 d after initiating incubation. At this time, the small tissue pieces were removed from the culture, and the adherent fibroblast-like cells were cultured to confluence, which subsequently took 2–3 weeks in culture. The cells were then trypsinized using 0.25% trypsin (Gibco-BRL) and passaged at 1×104 cells/cm2 in the medium described above. The cells were used after four passages.
P4 cells were cultured under conditions appropriate for inducing the differentiation of each lineage to investigate the differentiation potential of the fibroblast-like cells. Cells were seeded at a density of 3×103 cells/cm2 and the differentiation media were changed every 3–4 days. Osteogenic differentiation of MSCs assays were conducted in L-DMEM supplemented with 0.1 µM dexamethasone, 10% FBS, 0.2 mM ascorbic acid, and 10 mM β-glycerol phosphate (Sigma-Aldrich, St. Louis, MO, USA). Adipogenic assay was performed with high-glucose DMEM supplemented with 1 µM hydrocortisone, 0.5 mM 3-isobutyl-1-methylxanthine, 10% FBS, and 0.1 mM indomethacin (Sigma-Aldrich). Chondrogenic inductive medium consisted of 10% FBS, 3 ng/mL of TGF-β1, 0.2 mM ascorbic acid, 2 µg/mL of insulin, 30 ug/mL of sodium pyruvate, 0.1 umol/L of dexamethasone, 5 mM β-glycerin sodium phosphate, 0.4 mg/mL of bovine serum albumin, 2 ug/mlL of linoleic acid, and 2 ug/mL of alizarinic acid (Sigma-Aldrich).
Cells cultured without differentiation media served as the control group. UCMSCs were labeled with antibodies CD29, CD34, CD44, CD45, CD73, CD31, CD90, CD105, CD133, and corresponding isotype controls (from BD Biosciences), and were analyzed by flow cytometry (Cytometer 1.0, CytomicsTMFC500, Beckman Coulter Company, Brea, CA, USA).
Human 293T cells were maintained in DMEM with 10% FBS. Lentiviral vectors expressing short hairpin RNAs (shRNAs) against Ang1 (NM_001146) were synthesized as the following: forward: 5'-GAGGATCCCCGGGTACCGGTCGCC ACCATGA CAGTTTTCCTTTCCTTTG-3', and reverse: 5'-TCCTTGTAGTC CATA CCAAAATCTAAAGGTCGAATCATC-3'. The non-silencing shRNA sequences were used as negative controls and synthesized as follows: forward, 5'-CCGGTTCT CCGAACGTGT CACGTCTCGAGACGTGACACGTTCGGAGAATTTTTG-3', and reverse, 5'-AATTCAAAAATTCTCCGAACGTGTCACGTCTCGA GACGTGACAC GTTCGGAGAA-3', obtained from Gene Chem Co., Ltd (Shanghai, China). The coding sequence of human Ang1 (1497bp) was checked from NCBI. For over-
expression of Ang1, the open reading frame of Ang1 (NM-001146) was cloned into the lentiviral vector GV287 (Ubi-MCS-3FLAG-SV40-EGFP; Gene-Chem Co., Ltd). Information on vector GV287 is provided in Fig. 3A.
For in vivo study, P4 MSCs (1×105/mL) were transduced with Ang1 plasmid or empty vector plasmid at a multiplicity of infection of 8. The expression of Ang1 in UCMSCs was observed on a fluorescence microscope after 48 hours of lentiviral infection via fiuorescent microscopy and flow cytometry, and cells were harvested for western-blotting analysis to detect the protein expression of Ang1.
Sprague-Dawley rats (male, eight weeks old, and weight 200±20 g) were purchased from the Beijing Hua-Fukang Laboratory. All experimental protocols were approved by the animal Ethics Committee of Shandong University. The rats were randomized into a control group with normal saline (NS), LPS group, fibroblasts group, UCMSCs group (LPS+UCMSCs), and UCMSCs-Ang1 (LPS+UCMSCs-Ang1) treated group (n=25 for each group). Except the control group, ALI model groups were induced by the injection of LPS from E. coli 055:B5 (Sigma-Aldrich) (10 mg/kg of intra-peritoneal). and after 1 hour, the four groups were given 300 µL of NS , adult human lung fibroblasts (MRC-5) (5×105 cells in 300 µL of NS), UCMSCs-green fluorescent protein (GFP) (5×105 cells in 300 µL of NS) or UCMSCs-Ang1 (5×105 cells in 300 µL of NS) via injection into the tail vein respectively. GFP was used as a report gene to trace the UCMSCs. Five rats from each group were sacrificed at different points (6 hours, 24 hours, 48 hours, 8 days, and 15 days post-injection of LPS) and serum, lung tissue, and bronchoalveolar lavage fluid were harvested for cytokine measurements, as well as the assessment of lung histology and lung injury.
The right lower lung lobes of animals were used for histological study. Five-µm thick sections were obtained from paraffin-embedded lungs and stained with hematoxylin and eosin for histological study. Lung injuries were scored using the following categories: neutrophil infiltration into the airspace or vessel wall, alveolar congestion, vascular dilatation, hemorrhage, and thickness of alveolar wall/hyaline membrane formation. The category was graded on a 0 to 4 point scale: 0=no injury; 1=injury up to 25% of the field; 2=injury up to 50% of the field; 3=injury up to 75% of the field; and 4=diffuse injury.9
The trachea was isolated firstly, and then, the right bronchial tube was ligated after the rat was euthanized. The left lung was lavaged three times in situ with 3 mL of sterile NS at room temperature, and the recovered fluid was pooled. Cells, including neutrophils, were counted with a hemocytometer, and the BALF was centrifuged (3 kg, 20 min). The supernatant was frozen (-80℃) until ready for further analysis.
Myeloperoxidase (MPO) activities in the homogenized lung tissues were evaluated to quantify the neutrophil infiltrations.10 The lung tissues were homogenized in a phosphate buffer (20 mM, pH 7.4) and then centrifuged (at 30 kg for 30 min) after thawing. The pellet was resuspended in a potassium phosphate buffer (50 mM, pH 6.0) with 0.5% hexadecyltrimethyl ammonium bromide. The samples were centrifuged (at 20 kg for 15 min at 4℃). The absorbances were measured by spectrophotometry at 460 nm 0.0005% hydrogen peroxide and 0.167 mg/mL of O-dianisidine hydrochloride were added to each sample. The results were expressed as units of MPO per gram of wet tissue.
The right upper lobes were used for quantifying the magnitude of pulmonary edema. They were placed into previously-weighed microcentrifuge tubes and weighed. The lungs were desiccated under a vacuum at 80℃ for 24 hours and weighed again. The wet lung mass was divided by the dry lung mass to give the wet-dry ratio.
The levels of IL-10, TGF-β1, TNF-α, and IL-6 (ebioscience, USA) in serum (from blood drawn by cardiac puncture, and centrifuged for 10 min at 2 kg) were measured by ELISA according to the manufacturer's instructions.
Five groups of rats (n=10 per group) were used for survival study. LPS, fibroblast cells, UCMSCs, and UCMSCs-Ang1 were administered, respectively. Then, the rats were allowed to recover and the mortality was recorded till to 15 days after the treatment.
The lung tissues were cut into 5-µm thick sections and then observed by Laser confocal microscope. The distribution of UC-derived MSCs-Ang1 was analyzed by tracing GFP-positive cells. Twenty fields were randomly selected at ×40 magnification, and the GFP-positive and GFP-negative cells were counted. RT-PCR was used to detect of exogenous GFP mRNA expression in receptor lung tissue (Takara, Japan).
Analyses were done using SPSS 7.0 software (SPSS Inc., Chicago, IL, USA) and Graph-Pad Prism software (La Jolla, CA, USA). Data were shown as mean±SD. We used a one way ANOVA adjusted for multiple comparisons for statistical analysis. We used Kaplan-Meier survival curves and log rank to compare survival rates. In all instances, we considered values of p<0.05 to be statistically significant.
Most of the cells exhibited a spindle or irregular polygon shape that is consistent with MSCs (Fig. 1A and B). They expressed typical MSCs cell surface antigens, such as CD29, CD44, CD90, and CD105. Non-hematopoietic or endothelial cell markers, such as CD31, CD34, CD133, and CD45 could not be detected (Fig. 1C) The adherent cells induced to differentiate in culture exhibited multipotent differentiation potential, as shown by their ability to form osteoblasts and adipocytes. Osteoblasts were confirmed by alizarin red staining and alkaline-phosphate staining by the end of 14 days (Fig. 2A and B). In the normal growth medium, no mineralized matrix was observed in the control cells. Almost all cells contained numerous oil red-O-staining lipid droplets by the end of the second week (Fig. 2C and D). Cartilage structures were observed by immunohistochemical detection of cartilage specific type II collagen expression and toluidine blue staining after 2 weeks (Fig. 2E and F).
Results from functional differentiation experiments further indicated that the MSCs at passage 3 had strong adipogenesis, osteogenesis, and cartilaginous, respectively, under a given medium culture.
After obtaining enriched UCMSCs (P4), the cells were transduced with lentivirus vector carrying the GFP report gene and the Ang1 gene. The transduction efficiency was assessed by fluorescence microscopy of lentiviral infection. The transduction efficiency was about 95% (GFP-positive cells in 200 MSCs) at 72 h after UCMSCs-Ang1 tranduction. The photomicrographs of UCMSCs-Ang1 were obtained using a fluorescence microscope in the BX model at 72 h after transfection and prior to injection into the rats (Fig. 3B). At the same time, overexpression of Ang1 mediated by lentiviral transduction was confirmed in GFP cells by FACS sorting (Fig. 3C). Furthermore, the effectiveness of the lentiviral infection of LV-Ang1 was also confirmed by western-blot analysis. The protein expression of Ang1 in UCMSCs was significantly higher in the Ang1 overexpression group than that in the control group at 72 h after transfection (Fig. 3D).
After intra-peritoneal injection of LPS, the rats showed signs of systemic reaction, including piloerection, sneeze, lethargy, and diarrhea. Histological assessment of lung sections revealed that 6 hours after receiving LPS, capillary vessel engorgement, congestion, and abundant inflammatory infiltration in the lung stroma were observed diffusely, and some small abscesses and bullae of the lung also were found. These events peaked at the 48 hour time-point. UCMSCs-Ang1 animals displayed mild to moderate injury, compared to the MSCs group, and in the LPS group after 24 hours of receiving LPS, the severity was less significant (Fig. 4A). Five-level score evaluation system was used to evaluate the effect of UCMSCs or MSCs-Ang1 on lung injury. The lung injury score was lower significantly in the MSCs-Ang1 group at different time-points of 6 hours, 24 hours, 48 hours, 8 days, and 15 days (Fig. 4B). No improvement existed in the rats given injection of human fibroblast cell line in lung injury.
LPS was administered to rats after an injection of NS, UCMSCs, UCMSCs-Ang1, or fibroblasts. The total inflammatory cells counts in the BALF were attributable to an increase in neutrophils at 6 hours, 24 hours, 48 hours, and 8 days (Fig. 5A). Treatment with UCMSCs-Ang1 further reduced the BALF neutrophil counts at 24 hours and 48 hours, and neutrophils were similar to the NS group at 8 days and 15 days. LPS increased MPO activity significantly in lung tissues after ALI. These increases in the UCMSCs-Ang1 group reduced significantly, compared to UCMSCs, at 24 hours, 48 hours, 8 days, and 15 days (Fig. 5B).
LPS challenge produced obvious lung edema in the LPS group, as shown by increased lung wet-dry ratio. UCMSCs-Ang1 treatment attenuated the increase of the lung wet-dry ratio significantly, compared with the UCMSCs group, at 48 hours and 8 days after LPS was administered. This change took place in UCMSCs, compared with LPS, at 24 hours after ALI (Fig. 6).
In the MSCs-Ang1 treated group, the serum levels of TNF-α, IL-6, and TGF-β1 decreased significantly, compared to the other two groups; however, that of anti- inflamematory IL-10 increased.
In addition, in the UCMSCs-Ang1 group, the response of IL-10 peaked at 48 hours after injection of LPS, and decreased gradually thereafter. The increasing serum levels of IL-10 appeared to be no different among the four groups. This change in the concentration of IL-10 was not altered by administration of UCMSCs, expect for that at 24 hours and 48 hours (Fig. 7).
There was a significant improvement in survival rates between UCMSCs and UCMSCs-Ang1 groups. The 15-day survival rate of the UCMSCs group was 50% (5/10), while that of the UCMSCs-Ang1 group reached 70% (7/10). The differences among various groups were significant (p=0.0141). Treatment with UCMSCs-Ang1 engraftment significantly decreased mortality after ALI (Fig. 8).
At 6 hours after reconstitution, thin, flat, GFP-positive cells appeared in the parenchyma of UCMSCs-Ang1-treated recipient lungs (Fig. 9A), but not in the UCMSCs-treated lungs (Fig. 9B). With the extension of time, the GFP-positive cells tended to increase gradually in alveolar wall, capillary walls, and alveolar space. Until 8 days after LPS injection, cell quantities peaked. In the UCMSCs-Ang1 group, the positive rate of GFP cells in the pathological slices of the lung tissues was up to about 21% by fluorescence microscope, while the proportion in the UCMSCs group was 10% (Fig. 9C). The survival of GFP- positive cells was still visible on day 15, which indicated that reconstitution with GFP-expressing stem cells was successful.
At 8 days and 15 days after transplantation, GPF mRNA expression of UCMSCs-Ang1 group was statistically significantly higher than that in the UCMSCs group by RT-PCR (Fig. 9D and E). Although there was a similar phenomenon in early ALI, no statistical difference existed in the analysis of GPF mRNA levels.
In this study, recombinant lentivirus vector carrying GFP or Ang1 was successfully constructed and efficiently infected in UCMSCs by transfection technique, and Ang1 transfected UCMSCs resulted in a beneficial therapeutic effect in the treatment of LPS-induced lung injury. However, the administration of UCMSCs or fibroblast alone had little effect. The experiment suggested that the administration of UCMSCs carrying the Ang1 gene significantly improved both alveolar inflammation and permeability of lung injury evoked by LPS. All these data suggested that the severity of ALI was reduced and survival improved in the animals treated with UCMSCs-Ang1.
Several previously reports about BM-MSCs811 or Ang112 in the treatment of lung injury are available, and stem or progenitor cells are reported to restore function of damaged tissue in various preclinical disease models.1314 Based on our previously study by Li, et al.6 intravenous injection of UCMSCs clearly increased the survival rate of rats suffering from LPS-induced lung injuries and reduced systemic and pulmonary inflammation, and a study by Xu, et al.8 reported that lung injury induced by LPS was alleviated by treatment with MSCs (from bone marrow) carrying Ang1 (BM-MSCs-Ang1), while the UCMSCs carrying Ang1 was uncertain.
Gharib, et al.3 reported that activation of the endothelial cells (ECs) in response to inflammatory cytokines was tightly regulated by Ang1 in LPS-induced lung injuries, which was likely associated with toll-like receptor 4 (TLR4) inducing the expression of inflammatory cytokines and up-regulated leukocyte adhesion molecules. Ang1, the principal agonist of tyrosine kinase (TEK), is a factor in EC survival and vascular stabilization and maintains endothelial permeability in normal postnatal vasculature. Several studies have demonstrated that Ang1 carries anti-inflammatory,15 anti-permeability,16 angiogenic,17 and endothelial protective characteristics.18 Hence, this study tried to detect wheather the UCMSCs-Ang1 was beneficial for ALI.
This study demonstrated that UCMSCs transected with Ang1 were moderately effective in reducing ALI in rats after cell treatment, attenuating systemic and alveolar inflammation and reducing expression of endothelial-selective adhesion molecules.19 The effect of UCMSCs with overexpression of Ang1, compared with UCMSCs alone, not only on the lung edema and inflammatory cells infiltration, but also on the levels of various inflammatory cytokines was improved. Similarly with the study by Mei, et al.,1 who applied intra-tracheal instillation of endotoxin and systemic gene therapy, reported that systemic administration BM-MSCs with a lentiviral construct containing Ang1 could improve hemodynamics, reduce adhesion molecule expression, and prolong survival in mice with endotoxic shock. The mechanism might be closely related with the biologic effects of complex UCMSCs-Ang1. TEK receptors are largely restricted to the endothelium when combined with Ang1.15 Ang1 played a key role in contracting pulmonary capillaries, alleviating permeability, and inhibiting ECs apoptosis, while the effects of LPS on alveolar epithelium might not be directly modulated by Ang1.
LPS-induced ALI was closely related to up-regulation of adhesion molecules on the endothelial surface, which resulted in alveolar inflammatory response, leading to excessive trans-epithelial leucocyte migration into airspace, aggravation of epithelial injury, and increased pulmonary vascular leak.20 The hyperproduction of inflammatory mediators, such as IL-6, IL-8, and TNF-a, was considered as a direct response to lung injury. The inflammatory cytokine expression decreased in the MSCs alone.89 Similar to the above results, in this study, the serum levels of TNF-α, IL-6, and TGF-β1 decreased significantly in the MSCs-Ang1 treated group; however, that of anti-inflamematory IL-10 increased. Compared to the control group, the neutrophil counts in BALF that received UCMSCs and UCMSCs-Ang1 significantly decreased at earlier time points of 24 h and 48 h. Thus, the synergistic effect of Ang1 gene transfer may be attributed to alleviation of EC activation and, thus, the prevention of lung injury that depends on the recruitment of leukocytes from the pulmonary circulation into the injured lung. The possible mechanism of anti-inflammation needs to be further studied.
Survival rate is important to therapeutic efficacy. Here, the 15-day survival rate was 50% in the UCMSCs group and 70% in the UCMSCs-Ang1 group, although 40% in the LPS group. Moreover, the quantity of GFP positive cells peaked and a significant difference existed between both the UCMSCs-Ang1 and UCMSCs groups at 8 days after injury, which might indicate that the survival rate did not depend on the retention of UCMSCs and did not require long-term persistence of transplanted UCMSCs. Also, the cell engraftment rates were slightly higher than that of several previously studies reported few BMMSCs persistence after certain forms of injury.189 The decrease in survival rate might likely be associated with anti-inflammatory response to UCMSCs-Ang1, decrease in alveolar leakage, or even protection of systemic organs other than the lungs from inflammatory response.
In conclusion, UCMSCs-Ang1 could improve both systemic inflammation and alveolar permeability in ALI. UC-derived MSCs-based Ang1 gene therapy may be developed as a potential novel strategy for the treatment of ALI, representing an important therapy contributing to lower morbidity and mortality of critically ill patients. However, the possible mechanism by which genetically UCMSCs confer a therapeutic effect in lung injury remains to be studied.
Figures and Tables
 | Fig. 1The morphology and immunophenotype identification of UCMSCs. (A and B) Respectively by the inverted microscope and HE staining observation of MSCs (×100). (C) Immunophenotype of UC-derived MSCs. HE, hematoxylin and eosin; UCMSCs, umbilical cord-derived mesenchy mal stem cells. |
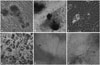 | Fig. 2Differentiation and identification of UCMSCs. (A) The formation of mineralized matrix shown by alizarin red staining (×200). (B) Alkaline phosphatase expression (×200). (C) Differentiation into adipocytes by the inverted microscope (×200). (D) Positive oil-red O staining (×200). (E) Immunohistochemical detection of cartilage specific type II collagen expression (×200). (F) Toluidine blue staining (×200). UCMSCs, umbilical cord-derived mesenchy mal stem cells. |
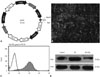 | Fig. 3(A) The information of vector GV287. Ubi-MCS-3FLAG-SV40-EGFP At a MOI=8, transduction of Ang1 into MSCs using a lentivirus vector. Viability of MSCs and high transduction efficiency (>95%) of GFP at 72 h after MSCs-Ang1 tranduction, ×200. (B) Fluorescence microscopy. (C) Flow cytometry. (D) The expression of Ang1 protein detection of transfected MSCs-Ang1 in western blotting. CON, control group with no transfection; NC (OE), negative control group transfected with GFP lentiviral vectors LV-GV287; OE, Ang1 overexpression group transfected with GFP and LV-Ang1. MOI, multiplicity of infection; GFP, green fluorescent protein. |
 | Fig. 4(A) Histological analysis indicated LPS injection caused capillary vessel engorgement, congestion, abundant inflammatory infiltration in lung stroma (×100). Administration of MSCs or MSCs-Ang1 improved the lung injury at all time points. (B) Lung injury score decreased significantly in the MSCs group and MSCs-Ang1 group at 6 hours, 24 hours, 48 hours, 8 days, and 15 days after LPS injection. (*p<0.05 compared with LPS controls; †p<0.05 comparing MSCs-Ang1 with MSCs). Fibroblast injections have no effect on improvement of both pathological morphology and lung injury score. NS, normal saline; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells. |
 | Fig. 5BALF neutrophil counts (A) and lung MPO activity (B). Neutrophil counts and lung tissue MPO activity were significantly higher in the LPS group, compared to the control group. Treatment with UCMSCs or UCMSCs-Ang1 significantly reduced LPS-induced increases in BALF neutrophil counts and lung MPO activity after 24 hours of acute lung injury (*p<0.05 compared with LPS controls; †p<0.05 comparing UCMSCs-Ang1 with UCMSCs). NS, normal saline; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells; BALF, bronchoalveolar lavage fluid; MPO, myeloperoxidase; UCMSCs, umbilical cord-derived mesenchy mal stem cells. |
 | Fig. 6Injection of UCMSCs-Ang1 reduced pulmonary edema in ALI. Pulmonary edema was measured as wet-dry ratio (*p<0.05 vs. the LPS group; †p<0.05 comparing UCMSCs-Ang1 with UCMSCs). NS, normal saline; LPS, lipopolysaccharide; UCMSCs, umbilical cord-derived mesenchy mal stem cells; ALI, acute lung injury. |
 | Fig. 7Levels of proinflammatory and antiinflammatory cytokines and chemokines in serum by ELISA. Increased levels of the pro-inflammatory cytokines TNF-α, IL-6, and TGF-β1 were down-regulated significantly comparing UCMSCs-Ang1 with UCMSCs group, which suggested Ang1 assistance anti-inflammatory effect in the early stage of inflammation (A, B, and C). In addition, the response of antiinflammatory IL-10 of UCMSCs-Ang1 group peaked at 48 hours after injection of LPS and decreased gradually at the following time points (D). The LPS-induced IL-10 increase appeared to be no different among the LPS and fibroblast groups (*p<0.05 vs. the LPS group; †p<0.05 comparing UCMSCs-Ang1 with UCMSCs). NS, normal saline; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells; ELISA, enzyme-linked immunosorbent assay; TNF, tumor necrosis factor; IL, interleukin; TGF, transforming growth factor; UCMSCs, umbilical cord-derived mesenchy mal stem cells. |
 | Fig. 8The survival rate of the MSCs-Ang1 group over 15 days is significantly higher than that of the MSCs group (70% vs. 50%; *p=0.0141). MSCs-Ang1 treatment improved survival rates at all time-points. NS, normal saline; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells. |
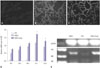 | Fig. 9Ang1 expressing UCMSCs labeled with the GFP were observed in lung sections from LPS-injured animals killed at 6 hours after acute lung injury. (A) UCMSCs-treated lungs. (B) UCMSCs-Ang1-treated recipient lungs. (C) UCMSCs-Ang1-treated recipient lungs at 8 days. RT-PCR results demonstrated that expression of GFP mRNA in MSCs-Ang1 group was higher than that in the MSCs group at 8 days and 15 days after ALI. (D) GFP mRNA relative quantitative analysized by RT-PCR at the designated time points. (E) At 8 days, UCMSCs-Ang1 expressed a greater quantity of GFP mRNAs than UCMSCs. 1–3 were respectively UCMSCs group, LPS group, UCMSCs-Ang1 group. GAPDH as the internal control (*p<0.05 compared with PBS control). GFP, green fluorescent protein; UCMSCs, umbilical cord-derived mesenchy mal stem cells; LPS, lipopolysaccharide. |
ACKNOWLEDGEMENTS
This research was supported by a grant of the Natural Scientific Fund of Shandong Province ZR2013HM001.
References
1. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007; 4:e269.

2. Gotts JE, Matthay MA. Mesenchymal stem cells and acute lung injury. Crit Care Clin. 2011; 27:719–733.

3. Gharib SA, Liles WC, Matute-Bello G, Glenny RW, Martin TR, Altemeier WA. Computational identification of key biological modules and transcription factors in acute lung injury. Am J Respir Crit Care Med. 2006; 173:653–658.

4. Slutsky AS, Hudson LD. PEEP or no PEEP--lung recruitment may be the solution. N Engl J Med. 2006; 354:1839–1841.

5. Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009; 110:1189–1197.

6. Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm (Lond). 2012; 9:33.

7. Paul A, Nayan M, Khan AA, Shum-Tim D, Prakash S. Angiopoietin-1-expressing adipose stem cells genetically modified with baculovirus nanocomplex: investigation in rat heart with acute infarction. Int J Nanomedicine. 2012; 7:663–682.
8. Xu J, Qu J, Cao L, Sai Y, Chen C, He L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008; 214:472–481.

9. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007; 179:1855–1863.

10. Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2011; 48:8–19.

11. Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014; 189:787–798.

12. Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010; 285:26211–26222.

13. Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006; 114:1 Suppl. I181–I185.

14. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004; 172:1266–1272.

15. Thurston G, Rudge JS, Ioffe E, Papadopoulos N, Daly C, Vuthoori S, et al. The anti-inflammatory actions of angiopoietin-1. EXS. 2005; (94):233–245.

16. Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000; 87:603–607.

17. Piao W, Wang H, Inoue M, Hasegawa M, Hamada H, Huang J. Transplantation of Sendai viral angiopoietin-1-modified mesenchymal stem cells for ischemic limb disease. Angiogenesis. 2010; 13:203–210.

18. McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007; 175:1014–1026.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download