Abstract
Purpose
During the late autumn to winter season (October to December) in the Republic of Korea, respiratory syncytial virus (RSV) is the most common pathogen causing lower respiratory tract infections (LRTIs). Interestingly, in 2014, human coronavirus (HCoV) caused not only upper respiratory infections but also LRTIs more commonly than in other years. Therefore, we sought to determine the epidemiology, clinical characteristics, outcomes, and severity of illnesses associated with HCoV infections at a single center in Korea.
Materials and Methods
We retrospectively identified patients with positive HCoV respiratory specimens between October 2014 and December 2014 who were admitted to Severance Children’s Hospital at Yonsei University Medical Center for LRTI. Charts of the patients with HCoV infection were reviewed and compared with RSV infection.
Results
During the study period, HCoV was the third most common respiratory virus and accounted for 13.7% of infections. Coinfection was detected in 43.8% of children with HCoV. Interestingly, one patient had both HCoV-OC43 and HCoV-NL63. Mild pneumonia was most common (60.4%) with HCoV, and when combined with RSV, resulted in bronchiolitis. Two patients required care in the intensive care unit. However, compared with that of RSV infection, the disease course HCoV was short.
Since finding severe acute respiratory syndrome (SARS) to occur due to infection by coronaviruses (CoVs),12 researchers have become more interested in human coronaviruses (HCoVs). Along with the outbreak of Middle East Respiratory Syndrome (MERS) in the Republic of Korea this year, HCoVs continue to impact global health.34 There are four respiratory HCoV species: 229E, NL63, OC43, and HKU1. In particular, subtypes 229E and OC43 are known to cause upper respiratory illness with relatively mild symptoms or even asymptomatic infections; however, these two subtypes still heavily impact the elderly and patients with cardiopulmonary disease.5
HCoV infections can occur anytime, anywhere, and in anybody. However, there are differences in geographic prevalence and age specificity. Among the four known respiratory HCoVs, typically only one, or at least a predominant type, is found at a particular time and in a particular area. In early childhood, OC43 and NL63 are detected at a younger age and more frequently.56
In the Republic of Korea, a single HCoV infection was detected every month in 2013, except June through September, in Seoul. The most detected virus was rhinovirus, and HCoVs were minor pathogens.7 From 2010 to 2012, HCoV-OC43 was the most prevalent subtype.8 Interestingly, however, in 2014, HCoV positive rates were lower than in other years, and HCoV caused fewer respiratory infections requiring admission, especially in children. While studies on HCoVs with lower respiratory tract infections (LRTIs) have been conducted in other countries,91011 there are no reports on HCoVs affecting LRTIs. In this study, we reviewed clinical presentations of HCoV infection during the 2014 winter season in Korea.
This study was performed at Severance Children’s Hospital in Seoul, Korea. Outpatient clinic, emergency room, and inpatient ward data were collected. From October 1 to December 31 in 2014, 504 patients under the age of 18 years with episodes of respiratory infection received nasopharyngeal swabs. All clinical data were collected by retrospective review of an electric medical record system.
We defined LRTIs as pneumonia or bronchiolitis. When a patient had abnormal lung sounds, such as rales or crackles, with local infiltration or consolidation on chest X-ray, we diagnosed the patient with pneumonia. When lower airway obstruction signs, such as wheezing, decreased lung sounds, or chest retractions, with either a normal or hyperinflation chest X-ray were present, we diagnosed the patient with bronchiolitis.
The swab specimens were sent to a virology laboratory in the Department of Laboratory Medicine at Yonsei University Medical Center for respiratory virus detection. DNA or RNA of respiratory viruses was extracted by TANBead Smart LabAssist-32 (BioKett, Taipei, Taiwan). Then the AdvanSure™ Respiratory Virus real-time RT-PCR Kit (LG Life Science, Seoul, Korea) was used to analyze all 504 samples. Using this kit, we were able to detect 14 types of viruses: respiratory syncytial virus (RSV) types A and B, influenza A and B, parainfluenza types 1, 2, and 3, rhinovirus A, metapneumovirus, HCoV-229E, HCoV-OC43, HCoV-NL63, and bocavirus.
A total of 504 nasopharyngeal swab samples were included in our study from October 1 to December 31, 2014. The positive rate for respiratory viruses was 67.9% (342 of 504 samples). All samples, except two that were obtained from expectorated sputum, were collected using nasopharyngeal swabs. During the study period, RSV was the most frequently detected virus (48.3%), and HCoV was the third most frequently detected virus (9.7%) regardless of subtype (Table 1). The peak incidence of HCoV in our hospital during the study period was during the 48th week of 2014, and this was similar to the pattern shown in the survey of Korean Centers for Disease Control and Prevention (KCDC; http://www.cdc.go.kr/CDC/info/CdcKrInfo0502.jsp?menuIds=HOME001-MNU1175-MNU0048-MNU0050) (Fig. 1). According to the plot, there was a certain amount of lag time between increases in nationwide HCoV infections and our hospital’s pediatric LRTIs due to HCoV.
Among the 342 positive samples, co-infection with two viruses was present in 71 samples and three viruses in six samples. When counting the co-infections, samples collected within a 4-week interval with the same result were counted as one result. The most frequent co-infection virus with HCoV was RSV (Table 2).
The most common signs of HCoV infection were fever and cough. Wheezing increased when combined with RSV. Gastrointestinal symptoms were not common.
Most of the patients had an abnormal chest X-ray, such as pneumonic infiltration, ground glass opacity, air trapping, and inter-costal lung herniation. Most of them had a lower respiratory tract infection, such as pneumonia, bronchiolitis, or bronchitis.
Antibiotics were used in almost all patients, because the viral panel result was obtained 2–3 days after admission. A nebulizer was less commonly used in the CoV only-infected group. Steroids were used more commonly in the CoV and RSV co-infected groups (Table 3).
We compared the fever day, obstruction signs (such as wheezing, chest retraction, and nasal flaring), hospital day (HOD), steroid use, and oxygen supply. As RSV infection is the major cause of LRTIs and hospital visits, we compared the selected parameters between RSV infection and HCoV infection. We did not compare the severity between each subtype of HCoV, because the patient population size was too small.
There were no differences among the two groups in terms of fever days, HOD, and oxygen supplementation. Obstruction signs, however, were more prevalent in the RSV only-infection group (51.9%) than in the HCoV-only infected group (25%; p=0.033). The RSV-infected group used more steroids than the HCoV-infected group (p=0.007) (Table 4).
As a percentage, HCoV among total respiratory viruses in the winter season was higher in 2014 than in 2013.
In 2013, among the total 254 tested samples, 180 samples were positive, and among these, 15 samples were positive for HCoV. In 2014, among the total 504 tested samples, 342 were positive, and among these, 48 samples were positive for HCoV. When these parameters were compared, there were no significant differences between them (Table 5).
Traditionally, CoV has been considered the most common cause of upper respiratory tract infection (URTI), which causes epidemics every 2–3 years. However, the emergence of SARS, caused by a group II CoV, has provided further insight that this virus can cause severe respiratory distress that is not simply limited to URTIs. In the 2000s, newly identified CoVs, HCoVNL63 and HCoV-HKU1, were introduced. CoV is now an important pathogen in pediatric infections, not only in URTIs but also in LRTIs, such as community-acquired pneumonia and bronchiolitis.12131415
Our data revealed a higher positive rate of total respiratory viruses.1617 As our hospital provides tertiary care, the disease severity of admitted children may be higher than other studies. Furthermore, viral study is performed only in children with suspected lower respiratory tract disease. This may have influenced higher detection rate of viruses.
Also, a higher positive rate of co-detection of RSV and CoVs was shown. Patterns of co-detection of respiratory virus differ from each study. In our report, patterns did not differ significantly from other reports.
Previous studies have reported that influenza virus follows RSV. However, our study showed that CoV seemed to follow RSV, as the influenza season followed an increase in CoV infections during the winter of 2014.
Interestingly in this study, there were no differences in fever days or days of hospital stay between RSV and HCoV. RSV is traditionally known as the most common cause of LRTIs in the winter season, especially in bronchiolitis. Since our hospital uses steroids to control obstruction symptoms, RSV showed more obstruction signs and that is why the RSV-infected group used more steroids than the HCoV-infected group. However, we expect that the disease courses of RSV and HCoV were similar in the winter of 2014, because the days of hospital stay and fever days were not very different.
When we compared years 2013 and 2014 in terms of HCoV, the parameters we thought could explain the severity of LRTI did not differ. While severity itself due to HCoV was similar, LRTIs caused by HCoV were much more common in 2014 than in 2013 (positive rate of HCoV 8.3% vs. 14.0%).
Interestingly, when the data from the Korea CDC showed an increase in HCoV, there was no detected HCoV in our pediatric patients. After about five weeks, HCoV began to be detected in our pediatric patients. It is difficult to determine the reason for the delayed HCoV detection in LRTIs, compared to that in general lower respiratory infections. This study is based on the respiratory virus infection data of the KCDC, which examines references weekly. These references are obtained from the sputum of adults and children who visit primary medical centers due to respiratory infection symptoms, not only for lower but also upper respiratory infection symptoms. This means that those viruses are thought to be the pathogens of the upper respiratory infection, as shown in Fig. 2. In general, respiratory viruses are spread by children who attend school. They transmit them to adults or infants and young children. In light on this result, we predict that when respiratory infections due to HCoV increase in primary care settings, as reported weekly by the KCDC, lower respiratory infections due to HCoV in children will increase within a few weeks.
During the last winter season, HCoV infection was common during the RSV infection period. Traditionally, RSV has been the main cause of bronchiolitis during this season, although, interestingly, HCoV also affected those with a similar diagnosis. Similar to other studies, we found the most common co-infection respiratory virus with HCoV was RSV.18 According to our data, bronchiolitis with airway obstruction is due to RSV not HCoV. On the other hand, HCoV induces pneumonia rather than bronchiolitis. This result was similar to that of previous studies.1011 Therefore, when caring the lower respiratory tract infection in the season when RSV and HCoV are both common, we should predict the causative virus. If bronchiolitis is the main presentation, RSV should be predicted, and if pneumonia is the main presentation, then HCoV should be predicted.
According to our hospital’s data, the distribution of HCoV subtype was not different than in other countries, such as the United States and those of Europe.1011 Regardless of the country, HCoV-OC43 and HCoV-NL63 are the main subtypes of HCoV for LRTIs.
In the Republic of Korea, respiratory infections due to CoV continue to be present every year. However, since lower respiratory infections due to CoVs were uncommon during the winter of 2014, clinicians did not have much interest in it. As we experienced last winter, CoV can be a pathogen associated with LRTIs of which clinicians should be aware. Although HCoV primarily causes URTIs, we should keep in mind that HCoV can cause both URTIs and LRTIs.
In conclusion, infections caused by HCoVs are common and can cause LRTIs. During an epidemic season, clinicians should be given special consideration thereto. When detections of HCoV are increasing, according to reports from the KCDC, pediatricians should keep in mind that LRTI due to HCoV will increase in 4–5 weeks. Also, when combined with other medical conditions, such as neurologic or cardiologic diseases, ICU care may be necessary.
Figures and Tables
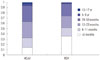 | Fig. 1Age distribution of HCoV and RSV from children <18 years old of age from October 2014 to December 2014. HCoV, human coronavirus; RSV, respiratory syncytial virus. |
 | Fig. 2Positive rate of HCoV among respiratory viruses. KCDC, Korean Centers for Disease Control and Prevention; Sev, Severance Children’s Hospital; HCoV, human coronavirus. |
Table 1
Viruses Identified in 504 Nasopharyngeal Swab Samples Obtained from Patients
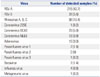
Table 2
Co-Infection Status of Respiratory Viruses

RSV, respiratory syncytial virus; PIV, parainfluenza virus; HCoV, human coronavirus; NA, not applicable.
One patient with RSV A and RSV B infection, one patient with HCoV-CO43 and HCoV-NL63 infection, one patient with RSV A, HCo-OC43, and rhinovirus, and one patient with RSV A, HCo-OC43, and Bocavirus was detected.
Table 3
Symptoms, Signs and Treatment among Patients Infected with HCoV

Table 4
Comparison of Disease Severity between HCoV and RSV in 2014 Winter Season
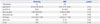
Table 5
Comparison of Disease Severity between HCoV in 2013 and 2014 Winter Seasons

References
1. Malave A, Elamin EM. Severe Acute Respiratory Syndrome (SARS)-lessons for future pandemics. Virtual Mentor. 2010; 12:719–725.

2. Cleri DJ, Ricketti AJ, Vernaleo JR. Severe acute respiratory syndrome (SARS). Infect Dis Clin North Am. 2010; 24:175–202.

3. Kim T, Jung J, Kim SM, Seo DW, Lee YS, Kim WY, et al. Transmission among healthcare worker contacts with a Middle East respiratory syndrome patient in a single Korean centre. Clin Microbiol Infect. 2016; 22:e11–e13.

4. Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015; 37:e2015033.

5. Mclntosh K, Englund JA. Coronaviruses and toroviruses, including severe acute respiratory syndrome. In : Cherry J, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez P, editors. Feigin and Cherry’s Textbook of pediatric infectious diseases. Philadelphia: Saunders Elsevier;2014. p. 2486–2495.
6. Dijkman R, Jebbink MF, Gaunt E, Rossen JW, Templeton KE, Kuijpers TW, et al. The dominance of human coronavirus OC43 and NL63 infections in infants. J Clin Virol. 2012; 53:135–139.

7. Ham H, Jang J, Jo S, Oh Y, Pak S. Infection frequency and mixed infection on eight viruses from patients with acute respiratory syndromes in Seoul. J Bacteriol Virol. 2014; 44:274–282.

8. Ham H, Jang J, Choi S, Oh S, Jo S, Choi S, et al. Epidemiological characterization of respiratory viruses detected from acute respiratory patients in Seoul. Ann Clin Microbiol. 2013; 16:188–195.

9. Chiu SS, Chan KH, Chu KW, Kwan SW, Guan Y, Poon LL, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005; 40:1721–1729.

10. Kristoffersen AW, Nordbø SA, Rognlien AG, Christensen A, Døllner H. Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr Infect Dis J. 2011; 30:279–283.

11. Lee J, Storch GA. Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J. 2014; 33:814–820.

12. Greenberg SB. Update on rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2011; 32:433–446.

13. Gallaher S. SARS: what we have learned so far. Dimens Crit Care Nurs. 2005; 24:51–54.
14. van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. 2004; 10:368–373.

15. Pyrc K, Berkhout B, van der Hoek L. Identification of new human coronaviruses. Expert Rev Anti Infect Ther. 2007; 5:245–253.

16. Liu T, Li Z, Zhang S, Song S, Julong W, Lin Y, et al. Viral Etiology of acute respiratory tract infections in hospitalized children and adults in Shandong Province, China. Virol J. 2015; 12:168.

17. Ge X, Han Z, Chen H, Cheng J, Gao M, Sun H. Characterization of acute respiratory infections among 340 infants in Wuxi, Jiangsu Province. Ann Transl Med. 2015; 3:264.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download