Abstract
Purpose
Our aim was to evaluate the efficacy and safety of oral beclomethasone dipropionate (BDP) in Korean patients with ulcerative colitis (UC).
Materials and Methods
The medical records of patients with active UC who were treated with BDP were retrospectively reviewed. Partial Mayo Clinic score (pMS) was calculated to determine disease activity. After 4 weeks of therapy, clinical remission, clinical response, and response failure rates were evaluated. Clinical remission was defined as a post-treatment pMS of 0 or 1, clinical response as a decrease of two of three points in pMS and >30% from baseline, and response failure as a lack of clinical response. Also, we considered that clinical remission was included in clinical response.
Results
Between July 2013 and April 2015, 95 patients with UC received BDP therapy at our institution (median age, 44 years; range, 12–81 years). After 4 weeks of therapy, clinical remission and clinical response rates were 50.5% and 73.7%, respectively. Mean change of pMS before and after BDP therapy was 2.4. There was no significant side effect reported. In multivariate analysis, disease activity was the only factor associated with a favorable response. Clinical remission rate was significantly higher in the mild disease activity group (66.7%) than that in the moderate or severe disease activity group (41.9%) (p=0.024).
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with repeated cycles of remission and relapse.1 UC requires life-long treatment both for induction and maintenance of remission.234 Corticosteroids are powerful medications that have been used for many years for the treatment of IBD. However, because of the toxicity profile of systemic steroids, clinicians often face challenges with using them for a longer term.
In recent years, new therapeutic agents for IBD have been introduced with more favorable profiles in terms of safety and toxicity, compared to traditional steroids.5 Topically acting oral steroids are a group of drugs characterized by a low systemic bioavailability due to first-pass liver metabolism that minimizes the systemic concentration of the drug.6 Budesonide (BUD) and beclomethasone dipropionate (BDP) are two steroids with low bioavailability that have been studied in the treatment of IBD. The European Crohn's and Colitis Organisation guidelines consider oral BUD as the first-choice drug for the treatment of mild-to-moderate Crohn's disease confined to the ileocecal area.7 Commercially available oral preparations of BUD have release systems that deliver BUD specifically to the distal ileum and proximal colon.5 Therefore, if BUD is taken orally, its efficacy fades out in the proximal colon. The distal colonic and rectal lesions do not benefit from this drug. Unlike oral BUD formulations, BDP was formulated as an oral enteric coated compound to be released in the distal part of the small bowel and throughout the colorectum owing to a combination of different methacrylic polymers that protect the drug from the action of the gastric fluid and then dissolve at specific pH values, targeting colonic inflammatory diseases.8 Data on the efficacy of oral BDP in IBD are quite limited, compared to those for BUD.9
The efficacy of oral BDP in UC has been assessed in several clinical trials and observational studies. Based on acquired evidence, BDP (5 mg/day) was recently released in Korea to treat mildly to moderately active UC. However, there have been no data regarding the efficacy of BDP in Korean patients with UC. Therefore, our aim was to evaluate the efficacy and safety of oral BDP in Korean patients with UC.
This is a retrospective, single-center study that included 95 patients with active UC who were treated with oral BDP tablets (5 mg/day) for 4 weeks. As endoscopy was not performed before and after BDP treatment in most of the patients, partial Mayo Clinic score (pMS; 0–9), the number of bowel movements (0–3), presence of blood in stools (0–3), and physician global assessment (0–3) was calculated to determine the disease activity.1011 Disease activity was defined as mild (pMS<5), moderate (pMS 5 to 7), and severe (pMS>7). After 4 weeks of therapy, clinical remission, clinical response, and response failure rates were evaluated. Clinical remission was defined as a post-treatment pMS of 0 or 1, clinical response as a decrease of two or three points in pMS and >30% from baseline, and response failure as a lack of clinical response. Also, we considered that clinical remission was included in clinical response. Disease extension was classified as E1, E2, and E3 according to the Montreal Classification.1213
Data regarding patient clinical characteristics, including disease duration and severity, concomitant therapies, and clinical outcomes were collected from reviewing the patients' hospital chart.
Patients undergoing treatment with 5-aminosalicylic acids (5-ASA) or sulfasalazine before the study were also included, and the use of these agents as concomitant treatments during the study period was permitted. Patients were excluded if they were on corticosteroids and were concomitantly diagnosed with severe cardiovascular, respiratory, hepatic or renal disease. Concurrent use of antidiarrheal drugs, topical mesalazine, and/or topical steroids was allowed. The only drug that was not permitted was a systemic oral or parenteral corticosteroid. Patients in whom pre-or post-treatment pMS could not be calculated were also excluded.
This study was performed in accordance with the Declaration of Helsinki and was approved by Institutional Review Boards of Severance Hospital.
Qualitative variables are expressed using frequencies. Continuous variables are expressed using mean±standard deviation. Fisher and chi-square tests were used to compare qualitative variables. Student's t-test was used to compare quantitative variables. The associations between variables and remission rate were further examined in multivariate model using logistic regression analysis. All tests were two-tailed with a significance level set at p<0.05. Analyses were performed using IBM SPSS software version 20.0 (Armonk, NY, USA).
The patients' clinical characteristics are summarized in Table 1. There were 95 evaluable patients, 47 men and 48 women. Their median age was 44 years (range, 12–81 years). Eighty-five patients (89.5%) had left-sided or extensive colitis, with a median disease duration of 68 months (range, 3–300; 68.4±59.4 months). Ninety-one patients (95.8%) had mild to moderate disease activity. All patients were on a maintenance therapy with oral or rectal 5-ASA compounds when the BDP therapy was started. For all patients, the BDP dose was 5 mg/day, which is the recommended regimen on the drug label.
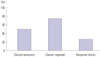
After 4 weeks of therapy, among the 95 patients, the clinical remission and clinical response rate were 50.5% (48/95) and 73.7% (70/95), respectively. Response failure was seen in 25 patients (26.3%) (Fig. 1). The mean change in pMS before and after receiving BDP therapy was 2.4 (±2.39) (p<0.001). In multivariate analysis, disease activity was the only factor associated with a favorable response (Table 2). Regardless of doses and types of 5-ASA, adding oral BDP was significantly effective in inducing clinical response or remission in Korean patients with UC (data not shown). The clinical remission rate was significantly higher in the mild disease activity group (66.7%) than that in the moderate or severe disease activity group (41.9%) (p=0.024) (Fig. 2). There was no significant difference in remission rates according to disease extension in our study. Moreover, no significant side effects were reported. Patients that stopped taking BDP did so because of a lack of efficacy, but not because of side effects. There were 17 patients that required rescue treatment in our study. Systemic steroids were used as a rescue therapy in 11 patients after BDP treatment failed. The other 6 patients were treated with infliximab or adalimumab, which are used for moderate-to-severe UC.
This is the first study reporting the efficacy and safety of oral BDP in Korean patients with active UC in clinical practice. In this study, more than 50% of the patients achieved clinical remission, and two-thirds of them showed a clinical response after oral BDP (5 mg/day) treatment for 4 weeks. A previously published RECLICU study showed that the remission rate was significantly higher in mild and moderate disease activity group than that in severe disease activity group. Generally, an oral BDP medication is recommended for UC patients with mild to moderate disease activity, as disclosed in the drug label. In our study, there were 4 patients with severe disease activity who used oral BDP. Similarly, the clinical remission rate was significantly higher in the mild disease activity group (66.7%) than that in the moderate or severe disease activity group (41.9%).
Unlike previous studies, in our study, all UC patients treated with BDP were included in the analysis to obtain more clinical and practical data on the efficacy and safety of oral BPD. UC patients in use of other concomitant medications and drug modification were not excluded at study inclusion. Patients with addition or dose increase of topical and/or oral 5-ASA were also included. The only drugs not permitted for concomitant use were systemic corticosteroids. All patients were on an oral 5-ASA maintenance treatment when the BDP therapy was started. A retrospective study revealed that maintenance treatment with thiopurine in UC patients was effective and safe.1415 There were 22 patients in this study who were concomitantly treated with azathioprine. Among the 22 patients, only 1 patient underwent BDP therapy with the addition of azathioprine. The other 21 patients were on a maintenance therapy of azathioprine when they started the oral BDP therapy. In subgroup analysis, there was no difference in clinical remission rates between the concomitant azathioprine use group and non-azathioprine medication group. There was only one patient on infliximab that started one day after BDP start. Thus, it might be possible that the use of concomitant medications contributes to clinical outcomes and increases he clinical remission and response rates. In clinical practice, many clinicians use oral BDP with other drugs, therefore, the role of concomitant medications on the results can be taken into consideration.
Two randomized clinical trials compared the efficacies of BDP and 5-ASA.1617 A 4-week, double-blind, placebo-controlled study demonstrated that oral BDP in combination with oral 5-ASA was significantly more effective in achieving clinical remission than 5-ASA alone in the treatment of patients with extensive or left-sided active UC.17 Subsequently, Campieri, et al.16 compared the efficacy of oral BDP (5 mg) and 5-ASA (2.4 g) in a 4-week multicenter, randomized, single-blind study. Oral BDP had an efficacy equivalent to that of 5-ASA in the treatment of left-sided and extensively mild-to-moderate UC. Moreover, patients with extensive UC were more likely to achieve a clinical and endoscopic improvement with oral BDP than with oral 5-ASA alone.16 The proportion of patients who reached a 4-week remission with oral BDP treatment was similar in both studies (58.6% and 63.0%, respectively).
Two studies, one prospective and one retrospective, evaluated the role and efficacy of BDP in clinical practice. The prospective study revealed that oral BDP therapy could be used instead of systemic corticosteroids in patients with mild-to-moderate UC who are not responding to 5-ASA and could be considered as a second-line treatment for these patients.18 The retrospective RECLICU study showed that oral BDP therapy induced a clinical response or remission in about two thirds of patients with active UC, with a remission rate of 44%. It had a good safety profile with mild adverse events in 434 patients. Remission rate was significantly higher in mild and moderate UC than in severe UC.19 Finally, Balzano, et al. compared the efficacy and safety of oral BDP and oral prednisone in mild to moderately active UC patients in an 8-week, multicenter, randomized, double-blind study. This was the first randomized controlled study confirming that oral topical BDP was non-inferior to prednisolone in the treatment of active UC, with oral BDP presenting less steroid-related adverse effects.20
We elicited post-treatment outcomes from the data that were collected at 4 weeks after completing the oral BDP therapy. We evaluated the pMSs at both 4 weeks and 8 weeks after the oral BDP treatment. After 4 weeks, clinical remission and clinical response rates were 50.5% and 73.7%, respectively. After 8 weeks, excluding the 9 patients who underwent rescue treatment at 4 weeks, the clinical remission and response rates for 86 patients were 63.9% and 70.9%, respectively. With continued use of 5-ASA and azathioprine, clinical remission could be achieved at 1 month after completing the oral BDP treatment. There were 17 patients who achieved clinical remission at 8 weeks, with partial clinical response or failure at 4 weeks. Thus, evaluating clinical outcomes at only 4 weeks is not recommended, as a clinical remission could be achieved slowly, even after discontinuing oral BDP.
There are some limitations to our study. First, there were no data in our study regarding the long-term treatment outcomes without the use of systemic corticosteroids. In a previously published single-center study, Papi, et al.18 found a remission rate of 75%, with more than 50% of the UC patients maintaining 1-year remission, with no further need of steroid treatment after the initial dose of 10 mg/day for 4 weeks and 5 mg/day for an additional 4 weeks. Second, as a retrospective study, there were only 16 patients that underwent endoscopy at the beginning of the oral BDP therapy.21 The aim of this study was to evaluate the efficacy and safety of oral BDP in Korean patients with UC in clinical practice. In this setting, endoscopy was not routinely performed. Third, in this study design, there was no control group. BDP is usually used concurrently with 5-ASA for remission induction. In our study, oral BDP was used in combination with 5-ASA for remission induction in all patients. Therefore, we were not able to compare the efficacy between with BDP and without BDP groups, and could not address the benefits of adding BDP in patients with mild disease activity UC having a generally favorable disease course. However, for remission induction, clinicians often face challenges while using systemic steroids for a long term because of the toxicity profile of systemic steroids. Oral BDP is a group of drugs that minimizes the systemic concentration of the drug with more favorable profiles, in terms of safety and toxicity, compared to traditional steroids. We think that here in lies the meaning of using oral BDP. In our study, BDP was efficacious in inducing a clinical response or remission in Korean patients with UC refractory to 5-ASA. More randomized control studies are needed to confirm the efficacy and safety of the drug.
In conclusion, oral BDP (5 mg/day for 4 weeks) was efficacious in inducing a clinical response or remission in Korean patients with UC. Patients with mild UC were more likely to induce remission than those with moderate or severe UC, after receiving the BDP treatment for 4 weeks. BDP had a good safety profile in these patients.
Figures and Tables
Table 1
Baseline Characteristics of the Patients

Table 2
Univariate and Multivariate Analysis of Factors Predictive of a Clinical Remission

ACKNOWLEDGEMENTS
This study was supported in part by the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number A111428, HI13C1345, HI12C0130). It was also supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1008096).
References
1. Lee HJ, Jung ES, Lee JH, Hong SP, Kim TI, Kim WH, et al. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with mild-to-moderate ulcerative colitis. Hepatogastroenterology. 2012; 59:1415–1420.
2. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014; 20:11525–11537.

3. Choi CH, Kim YH, Kim YS, Ye BD, Lee KM, Lee BI, et al. [Guidelines for the management of ulcerative colitis]. Korean J Gastroenterol. 2012; 59:118–140.

4. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14.

5. Saibeni S, Meucci G, Papi C, Manes G, Fascì-Spurio F. Low bioavailability steroids in inflammatory bowel disease: an old chestnut or a whole new ballgame? Expert Rev Gastroenterol Hepatol. 2014; 8:949–962.

6. Harris DM. Some properties of beclomethasone dipropionate and related steroids in man. Postgrad Med J. 1975; 51:Suppl 4. 20–25.
7. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010; 4:28–62.

8. Levine DS, Raisys VA, Ainardi V. Coating of oral beclomethasone dipropionate capsules with cellulose acetate phthalate enhances delivery of topically active antiinflammatory drug to the terminal ileum. Gastroenterology. 1987; 92:1037–1044.

9. Fascì Spurio F, Aratari A, Margagnoni G, Doddato MT, Chiesara F, Papi C. Oral beclomethasone dipropionate: a critical review of its use in the management of ulcerative colitis and Crohn's disease. Curr Clin Pharmacol. 2012; 7:131–136.

10. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.

11. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987; 317:1625–1629.

12. Choi JH, Kim ES, Cho KB, Park KS, Lee YJ, Lee SM, et al. Old age at diagnosis is associated with favorable outcomes in Korean patients with inflammatory bowel disease. Intest Res. 2015; 13:60–67.

13. Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19:Suppl A. 5A–36A.

14. Yamada S, Yoshino T, Matsuura M, Kimura M, Koshikawa Y, Minami N, et al. Efficacy and safety of long-term thiopurine maintenance treatment in Japanese patients with ulcerative colitis. Intest Res. 2015; 13:250–258.

15. Jeon SR, Kim WH. Is long-term therapy with thiopurines effective for maintaining remission in patients with moderate-to-severe ulcerative colitis? Intest Res. 2015; 13:191–192.

16. Campieri M, Adamo S, Valpiani D, D'Arienzo A, D'Albasio G, Pitzalis M, et al. Oral beclometasone dipropionate in the treatment of extensive and left-sided active ulcerative colitis: a multicentre randomised study. Aliment Pharmacol Ther. 2003; 17:1471–1480.

17. Rizzello F, Gionchetti P, D'Arienzo A, Manguso F, Di Matteo G, Annese V, et al. Oral beclometasone dipropionate in the treatment of active ulcerative colitis: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2002; 16:1109–1116.

18. Papi C, Aratari A, Moretti A, Mangone M, Margagnoni G, Koch M, et al. Oral beclomethasone dipropionate as an alternative to systemic steroids in mild to moderate ulcerative colitis not responding to aminosalicylates. Dig Dis Sci. 2010; 55:2002–2007.

19. Nunes T, Barreiro-de Acosta M, Nos P, Marin-Jiménez I, Bermejo F, Ceballos D, et al. Usefulness of oral beclometasone dipropionate in the treatment of active ulcerative colitis in clinical practice: the RECLICU Study. J Crohns Colitis. 2010; 4:629–636.

20. Van Assche G, Manguso F, Zibellini M, Cabriada Nuño JL, Goldis A, Tkachenko E, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Am J Gastroenterol. 2015; 110:708–715.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download