Abstract
Left atrial appendage (LAA) occlusion can be employed as an alternative treatment to oral anticoagulation in patients with atrial fibrillation to prevent embolic events. Atrial septal defect (ASD) may be related with right heart dysfunction and allow paradoxical embolism to occur. However, occlusion of both LAA through atrial access with ostium secundum ASD and ASD in the same setting is unusual. Therefore, we report a case in which a LAA and an ASD was sequentially occluded.
Left atrial appendage (LAA) occlusion is performed in patients with non-valvular atrial fibrillation (AF) to prevent embolic events as an alternative treatment to oral anticoagulation.12 Atrial septal defect (ASD) may cause right heart dysfunction and paradoxical embolism to occur. In patients with AF and ASD, occlusion both LAA and ASD in the same setting could be a possible strategy for an effective prevention of stroke and right heart failure. We report a case in which a LAA and ASD were sequentially occluded during the same procedure.
A 68-year-old male was referred for ASD closure, because the right ventricle (RV) was markedly enlarged. He had suffered from a persistent AF without significant valvular disease, but refused to take a long-term anti-coagulation {CHA2DS2VASc: 3 [congestive heart failure, hypertension, age ≥75 years (2 points), diabetes mellitus, prior stroke or transient ischemic attack (2 points), vascular disease, age 65 to 74 years, and female sex] and HAS-BLED: 2 [hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (>65 years), drugs/alcohol concomitantly]}. Recently, he was diagnosed with gastric cancer and treated with endoscopic resection. Simultaneous ASD and LAA occlusion were considered because LAA occlusion could be an alternative option of stroke prevention in AF and might be in trouble after ASD closure. Transthoracic echocardiography (TTE) showed a right atrium (RA) and RV with significant left to right shunt. Transesophageal echocardiographic (TEE) indicated a single oval shaped secundum defect of 12×6 mm (Fig. 1A), and no thrombus was detected in left atrium (LA) and LAA (Fig. 1C). The landing zones of LAA were 24 mm in 45°, 28 mm in 90°, and 28 mm in 135°.
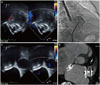
The procedure was performed under general anesthesia and TEE. A 6F multipurpose catheter (A&A M.D., Seoul, Korea) with a 0.035-inch hydrophilic wire (Terumo, Tokyo, Japan) was passed through the ASD under 3D TEE guidance, and a 0.035-inch Amplatz super stiff guidewire (Boston Scientific Corp., Natick, MA, USA) positioned pulmonary vein. Because the anatomy of the LAA was toward the anterio-superior direction and not complex, ASD could be used as atrial septal access for LAA occlusion. To shorten the procedure time, the ASD was measured 11 mm with 24 mm sized Amplatzer sizing balloon (AGA Medical Corp., Golden Valley, MN, USA) (Fig. 1B). Then, the catheter was replaced by a 13 French Amplatzer TorqVue delivery sheath (St. Jude Medical, St. Paul, MN, USA) over a 0.035-inch stiff wire. Contrast injection through this sheath with a 5F pig-tail catheter was used to depict the anatomy and the size of the LAA (Fig. 1D). The maximal LAA size was 28 mm on LA angiography. Based on measurement, a 30-mm Amplatzer cardiac plug (ACP, St. Jude Medical) was chosen and successfully occluded the LAA (Fig. 2A and B). After releasing the ACP, an 11-mm Amplatzer septal occluder (St. Jude Medical) was introduced through the same delivery sheath and deployed successfully (Fig. 2C). Placement was confirmed by TEE, the device was released, and the delivery sheath was removed. Angiogram after the procedure revealed no atrial communication.
Before discharge, correct device positions were confirmed by a TTE and computerized tomography (Fig. 2D). The patient was discharged the following day, with an antithrombotic regimen consisting of Apixaban 5 mg twice a day.
We checked a follow-up TEE to confirm proper seating of the devices and to look for thrombi or residual leak at 2 months. It showed proper position of both devices and neither thrombi nor leakage. Then, the patient discontinued Apixaban and changed to Aspirin and Clopidogrel.
The main finding of our case was the use of the ASD as the access for LAA occlusion and to close simultaneously both a LAA and ASD. In this procedure, it was difficult to use the ASD as the access route for LAA occlusion due to its more cranial position in the interatrial septum and relative small size of ASD. However, we could successfully cannulate the ASD with better supportable 6F multipurpose catheter and 3D TEE guidance. Koermendy, et al.3 reported a 96% success of LAA occlusion through ASD or patent foramen ovale as an access of atrial septum. Avoiding a transseptal puncture can obviate related complications, such as cardiac tamponade and perforation of adjacent organs.4 Another advantage of avoiding the transseptal puncture is not to create an iatrogenic septal defect. Although a persistent iatrogenic ASD was found in only 6.8% of patients who underwent LAA closure at 12 months, which could potentially mediate paradoxical embolism, pulmonary hypertension, or right ventricular dysfunction.56
However, ASD may allow paradoxical embolism to occur due to communication between the venous and systemic circulation. 789 ASD is a risk factor for stroke, which is only increased by the presence of AF.79 An ASD closure has been recommended if the RA or RV are enlarged with significant left to right shunt. Patients with small shunts and normal RV size are generally asymptomatic and require no medical, interventional therapy.10 In current case, ASD closure was indicated because of right atrial and RV enlargement. However, the patient had persistent AF and gastrointestinal malignancy, which could increase a bleeding risk. In addition, the patient refused long-term anti-coagulation due to an increase in bleeding tendency. In the clinical scenario, closure of ASD subsequent to LAA occlusion may be the best strategy for the patients. After ASD closure, LAA occlusion can be possible through the septal puncture outside of the ASD closure device, which may increase the periprocedural complications. For the possibility of early embolization of the LAA occluder device into the LA, ASD closure is recommended as a staged procedure or at least 10 minutes after LAA occlusion in the same setting.11 However, if embolization of the LAA occluder may occur after ASD closure, we can puncture the septum around the pre-implanted ASD device and retrieve an embolized device.
Above all, the patient was concerned about anticoagulation and consequential increased gastrointestinal bleeding because of previous endoscopic mucosal resection due to early gastric cancer. Therefore, it was an applicable strategy in the patient with secundum ASD and AF that closure of both the interatrial communication and LAA.
In conclusion, simultaneous occlusion of ASD followed by the LAA though the ASD is a safe and effective way to prevent stroke in patients with non-valvular AF and a high risk of bleeding.
Figures and Tables
Fig. 1
Two dimensional TEE image of ASD (A). Fluoroscopic evaluation of ASD during expansion of the sizing balloon (B). TEE (C) and fluoroscopic images (D) of left atrial appendage before implantation. ASD, atrial septal defect; TEE, transesophageal echocardiographic.
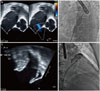
Fig. 2
The deployed left atrial appendage closure device (ACP 30 mm) was confirmed with transesophageal echocardiographic imaging (arrow: left circumflex artery) (A) and fluoroscopic imaging (B). The Amplatzer septal occluder (11 mm) was located in the proper position, which was confirmed by transesophageal echocardiography (C). After the procedure, implanted Amplatzer septal occluder (11 mm) and ACP (30 mm) demonstrated by heart computed tomography (D). ACP, Amplatzer Cardiac Plug.
ACKNOWLEDGEMENTS
This study was supported by the Cardiovascular Research Center, Seoul, Korea and Dr. Jung-Sun Kim is a consultant for Amplatzer cardiac plug, St. Jude Medical.
References
1. Kim YL, Joung B, On YK, Shim CY, Lee MH, Kim YH, et al. Early experience using a left atrial appendage occlusion device in patients with atrial fibrillation. Yonsei Med J. 2012; 53:83–90.

2. Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace. 2014; 16:1397–1416.

3. Koermendy D, Nietlispach F, Shakir S, Gloekler S, Wenaweser P, Windecker S, et al. Amplatzer left atrial appendage occlusion through a patent foramen ovale. Catheter Cardiovasc Interv. 2014; 84:1190–1196.

4. Tzeis S, Andrikopoulos G, Deisenhofer I, Ho SY, Theodorakis G. Transseptal catheterization: considerations and caveats. Pacing Clin Electrophysiol. 2010; 33:231–242.

5. Alkhouli M, Sarraf M, Zack CJ, Holmes DR, Rihal CS. Iatrogenic atrial septal defect following transseptal cardiac interventions. Int J Cardiol. 2016; 209:142–148.

6. Singh SM, Douglas PS, Reddy VY. The incidence and long-term clinical outcome of iatrogenic atrial septal defects secondary to transseptal catheterization with a 12F transseptal sheath. Circ Arrhythm Electrophysiol. 2011; 4:166–171.

7. Berthet K, Lavergne T, Cohen A, Guize L, Bousser MG, Le Heuzey JY, et al. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke. 2000; 31:398–403.

8. Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007; 357:2262–2268.

9. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988; 318:1148–1152.

10. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008; 52:e143–e263.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download