Abstract
Purpose
Glucocorticoids, stress-related hormones, inhibit hair growth. Intracellular glucocorticoid availability is regulated by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). 11β-HSD1 was recently detected in keratinocytes and fibroblasts. However, the expression of 11β-HSD1 in human hair follicles remains unknown. We aimed to examine 11β-HSD1 expression in human dermal papilla cells (DPCs) and to investigate whether modulation of 11β-HSD1 activity can regulate the negative effects of glucocorticoids on DPCs.
Materials and Methods
11β-HSD1 expression in normal human scalp skin was examined by immunohistochemistry. 11β-HSD1 protein was detected in Western blots of human DPCs. Cultured human DPCs were treated with cortisol with or without a selective 11β-HSD1 inhibitor and subsequently stained for Ki-67 antibody. Expression levels of 11β-HSD1, Wnt5a, alkaline phosphatase (ALP), and vascular endothelial growth factor (VEGF) were analyzed by Western blotting.
Results
11β-HSD1 was detected in dermal papilla in human scalp skin by immunohistochemistry. Human DPCs expressed 11β-HSD1 protein in vitro. Furthermore, cortisol stimulated the expression of 11β-HSD1 in DPCs. Glucocorticoids decreased cellular proliferation and the expression of Wnt5a, ALP, and VEGF in DPCs. A specific 11β-HSD1 inhibitor significantly attenuated the anti-proliferative effects of cortisol and reversed the cortisol-induced suppression of Wnt5a, ALP, and VEGF expression in DPCs.
Sustained exposure to stressful environments or psychological stress has been reported to trigger and exacerbate a number of skin disorders.1 Previous clinical studies have suggested that stress is a potential trigger for various types of hair loss, such as telogen effluvium, androgenetic alopecia, and female pattern hair loss.23 Chronic stress has been shown to exert a profound inhibitory effect on hair growth in a stress mouse model and in organ-cultured human scalp hair follicles.45 Moreover, a mouse model of alopecia areata and alopecic monkeys have been reported to have an increased hypothalamic-pituitary-adrenal (HPA) axis tone centrally and peripherally in hair follicles.67 Stress-related neurohormones of the HPA axis, including corticotropin-releasing hormone, adrenocorticotropic hormone, and cortisol, play important roles in the stress response, and hair follicles appear to be a functional peripheral equivalent of the HPA axis.5 Glucocorticoids are major players in the adverse consequences of stress, and blockage of glucocorticoids action can reverse psychological stress- and exogenous glucocorticoids-induced skin structure and function defects.89 Previous in vivo experiments have demonstrated that dexamethasone induces hair follicle regression.1011 Moreover, a recent study demonstrated that glucocorticoids inhibit the proliferation of dermal papilla cells (DPCs) by inducing cell cycle arrest and also suppress the expression of growth factors, which are important mediators of hair follicle growth in DPCs.12 Unlike circulating inactive glucocorticoids that bind to corticosteroid-binding globulins, intracellular glucocorticoids are converted to an inactive form or an active form by isoenzymes of 11β-hydroxysteroid dehydrogenase (11β-HSD) before they act on glucocorticoid receptor. 11β-HSD type 1 (11β-HSD1) is predominantly a reductase that converts inactive cortisone to active cortisol, whereas 11β-HSD type 2 (11β-HSD2) catalyzes the reverse reaction.13 In addition to liver, lung, adipose tissue, ovaries, and the central nervous system, 11β-HSD isoforms are also expressed in skin.1415 11β-HSD1 is abundantly expressed in keratinocytes, fibroblast, and sebocytes. In contrast, 11β-HSD2 is expressed in sweat glands, but not in keratinocytes.14 By prereceptor regulation of active cortisol level in tissues, 11β-HSD1 has been demonstrated to be involved in cell proliferation, wound healing, inflammation, and aging in skin.16 11β-HSD1 was detected in the outer root sheath (ORS) of hair follicles in mouse skin by immunohistochemical staining.14 However, the expression and localization of 11β-HSD1 in the epidermal and dermal compartments of human hair follicles have not been studied in detail. Dermal papilla are the major dermal compartments of the hair follicle and play an important role in the regulation of hair development, growth, and cycling.17 In this study, we investigated the expression and regulation of 11β-HSD1 in human DPCs in vitro and in vivo. Additionally, we examined whether inhibition of 11β-HSD1 activity could modulate the inhibitory effects of glucocorticoids on the proliferation of DPCs and the expression of anagen follicle markers and growth factors in DPCs.
A total of 12 paraffin-embedded human scalp skin samples were obtained from patients undergoing excision for benign tumors of the scalp. For immunohistochemical staining, primary rabbit polyclonal anti-11β-HSD1 antibody (1:100 dilution, Abcam, Cambridge, UK) was reacted overnight at 4℃, and a horseradish peroxidase-conjugated secondary antibody was added to sections for 1 hour at room temperature. All sections were lightly counterstained with hematoxylin. This study was approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, and all skin samples were obtained after receiving written informed consent from the donors (IRB No. 3-2015-0034).
DPCs of human origin were purchased from PromoCell GmbH (Heidelberg, Germany) and maintained in PromoCell growth medium. Cells were incubated at 37℃ in an atmosphere containing 5% CO2. Cells from passages 2 or 3 were used in experiments. DPCs were seeded in 100-mm culture dishes and preincubated for 24 hours before treatment with test materials. Cortisol was purchased from Sigma Aldrich (H6909, St Louis, MI, USA). A selective inhibitor of 11β-HSD1, 385581, was obtained from Merck (Darmstadt, Germany) and was dissolved in dimethyl sulfoxide (DMSO) and used at a final concentration of 0.1 µM (1:100000 dilution) in cell culture medium.
Cultured DPCs grown on coverslips were fixed in 4% paraformaldehyde, and endogenous peroxidase activity was blocked by soaking the slides in 2% hydrogen peroxide for 10 min. After rinsing in Tris-buffered saline containing 0.1% bovine serum albumin, slides were incubated overnight at 4℃ with anti-Ki-67 antibody at a dilution of 1:100. After washing in phosphate-buffered saline (PBS), the specimens were incubated with fluorescein-conjugated secondary antibody (1:200) for 1 hour. 4′,6′-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining. The number of Ki-67-expressing cells was determined by counting five non-overlapping high power fields (×200) captured by confocal microscopy in each group and expressed as a percentage of the total number of DAPI-stained nuclei.
Treated DPCs were dissolved in PRO-PREP™ protein extraction solution (iNtRON Biotech, Seongnam, Korea). Total cell lysates (30 µg) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA) using Towbin buffer. Membranes were then blocked with 5% milk in TBS + 0.1% Tween-20 (Sigma Aldrich, Dorset, UK) and incubated with anti-11β-HSD1 antibody (1:500 dilution, Abcam), anti-vascular endothelial growth factor (VEGF) antibody (1:500 dilution, Abcam), anti-Wnt5a antibody (1:500 dilution, Abcam), or anti-alkaline phosphatase (ALP) antibody (1:500 dilution, Abcam) as primary antibodies and monoclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:1000, Beyotime Institute of Biotechnology, Nanjing, China) as a control. Blots were reacted with Immobilon Western reagent (Millipore) and detected using an Amersham Hyperfilm electrochemiluminescence (ECL) assay (GE Healthcare, Buckinghamshire, UK). Signals were detected using the ECL Plus Western blotting detection system (Amersham, Buckinghamshire, UK).
First, immunohistochemistry was performed to confirm that 11β-HSD1 is expressed in human scalp skin and hair follicles. Consistent with previous studies, 11β-HSD1 was expressed in the interfollicular epidermis and ORS of hair follicles (Fig. 1A). Expression of 11β-HSD1 was also observed in the matrix keratinocytes of the hair bulb and DPCs (Fig. 1).
11β-HSD1 antibody recognized a single band of approximately 38 kDa in lysates of cultured human DPCs by Western blot, indicating the expression of 11β-HSD1 protein by cultured human DPCs (Fig. 2). Glucocorticoids have been shown to modulate 11β-HSD1 expression in various cell lines and tissues; therefore, we examined the effect of a glucocorticoid on the expression of 11β-HSD1 in cultured human DPCs. Treatment of cultured DPCs with 10-8 M cortisol for 24 and 48 hours had no significant effect on the expression of 11β-HSD1. However, 10-7 M cortisol stimulation for 24 hours induced a 1.7±2.5-fold significant increase in 11β-HSD1 protein expression, compared with unstimulated cells (Fig. 2).
A recent study showed that glucocorticoids inhibit the proliferation of DPCs through cell cycle arrest. Consistent with this previous report, treatment of cultured human DPCs with cortisol for 48 hours inhibited cellular proliferation as determined by Ki-67 staining. To investigate whether prereceptor regulation of glucocorticoid action by 11β-HSD1 inhibition could modulate the ability of cortisol to inhibit proliferation of DPCs, human DPCs were treated with 10-7 M cortisol for 48 hours with or without pretreatment with 100 nmol L-1 of a selective inhibitor of 11β-HSD1, and then cellular proliferation was assessed by Ki-67 staining. As shown in Fig. 3, 11β-HSD1 inhibitor pretreatment significantly reversed glucocorticoid inhibition of DPC proliferation. 11β-HSD1 inhibitor alone treatment had no effects on cellular proliferation.
Because DPCs play a critical role in control of hair growth and cycling, we next investigated whether inhibition of 11β-HSD1 could modulate the effect of glucocorticoids on the expression of dermal papilla anagen markers and growth factor, which is important in hair growth in DPCs, by Western blotting. Treatment of DPCs with 10-7 M cortisol for 48 hours strongly reduced the expression of dermal papilla anagen markers Wnt5a and ALP. In addition, in agreement with a previous report, cortisol stimulation of DPCs for 48 hours downregulated VEGF expression. To assess the involvement of 11β-HSD1 in the cortisol-induced decrease in expression of dermal papilla anagen markers and growth factor, we examined the effect of an 11β-HSD1 inhibitor. Fig. 4 shows that the addition of 100 nmol L-1 11β-HSD1 inhibitor to the culture media 30 minutes prior to cortisol treatment significantly attenuated the cortisol-induced downregulation of Wnt5a, ALP, and VEGF expression in DPCs. 11β-HSD1 inhibitor alone did not affect the expression of Wnt5a, ALP, and VEGF in DPCs.
Glucocorticoids have been reported to exert deleterious effects on the growth of hair follicles and hair cycling,51011 suggesting that glucocorticoids have a role in stress-associated hair loss. However, the expression and functional role of the cortisol-activating enzyme 11β-HSD1 in human hair follicles have not yet been elucidated. Here, we first demonstrated that 11β-HSD1 is expressed in human DPCs both in situ and in vitro at the protein level. 11β-HSD1 was also detected in ORS and hair matrix cells in the bulb of the hair follicle in our immunohistochemistry analysis of human scalp samples. These results confirm that 11β-HSD1 is expressed in both epithelial and dermal compartments of human hair follicles, as well as epidermal keratinocytes and dermal fibroblasts.
Previous studies have demonstrated that 11β-HSD1 is upregulated in human dermal fibroblasts and human immortalized SZ95 sebocytes by glucocorticoid treatment,1415 indicating a positive feedback loop between the induction of 11β-HSD1 and the glucocorticoid receptor cycle in skin cells. Consistent with these previous studies, we demonstrated that 10-7 M cortisol treatment of DPCs for 24 hours significantly increased 11β-HSD1 protein expression. Based on a recent study that showed glucocorticoid receptor expression by human DPCs and our data, we hypothesize that DPCs are not only the target cells for glucocorticoids, but also metabolize and synthesize the active forms of glucocorticoids via the presence of 11β-HSD1.
DPCs are specialized mesenchymal cells in hair follicles that play a critical role in hair follicle morphogenesis, hair growth, and cycling via communication with the epithelial components.17 Previous studies have demonstrated that glucocorticoids decrease the proliferation of DPCs and the expression of growth factors for hair growth, such as VEGF and hepatocyte growth factor, and inhibit local insulin-like growth factor 1 availability in cultured DPCs.121819 We also confirmed the inhibitory effect of cortisol on the proliferation of DPCs and expression of VEGF. Our study further revealed that cortisol suppressed the expression of dermal papilla biomarkers involved in the maintenance of human dermal papilla properties, including Wnt5a and ALP. ALP is a ubiquitous dermal papilla marker. Wnt5a, a non-canonical Wnt family member, is expressed specifically in dermal papillae during early skin development.2021 A recent study showed that, in mouse vibrissa follicles, ALP levels were pronounced during the early anagen phase and diminished after the mid-anagen phase.22 It was also found that the hair inductivity of cultured DPCs and the expression of ALP decreased after passages in the same manner.2223 These findings suggest that ALP is an indicator of hair inductivity and a marker of the early anagen phase in DPCs. Wnt5a in DPCs is important for the regulation of hair cycling and growth and maintenance of hair follicle-inductive properties, suggesting that Wnt5a plays an important role in maintaining the intrinsic properties of DPCs.24 Taken together, our results indicate that glucocorticoids not only inhibit the proliferation of DPCs, but also affect dermal papilla properties and can promote the transition of hair follicles from anagen to telogen phase. These findings are in agreement with a previous study that used a murine model to demonstrate that dexamethasone can induce the catagen phase in murine hair follicles.10
In addition to circulating glucocorticoid levels, the action of glucocorticoids at the cellular level is important. Tissue-specific metabolism of glucocorticoids by 11β-HSD1 is critical in regulating the development of the adverse effects associated with glucocorticoids. Therefore, targeting 11β-HSD1 can offer a new strategy to control the conditions associated with excess glucocorticoids. 11β-HSD1 is highly expressed in metabolic tissues, and selective 11β-HSD1 inhibitors have been developed to control various metabolic features and tested in phase II studies for iatrogenic Cushing's disease and idiopathic intracranial hypertension.2526 Recently, inhibition of 11β-HSD1 was demonstrated to prevent age-related skin changes and to reverse stress- and glucocorticoid-induced delays in cutaneous wound healing.27 In this study, we demonstrated that 11β-HSD1 in DPCs plays an important role in modulating glucocorticoid action in DPCs. Pharmacological inhibition of 11β-HSD1 by a selective inhibitor significantly attenuated the glucocorticoid-induced inhibition of proliferative activity and reduced VEGF expression in DPCs. Moreover, we observed that the selective 11β -HSD1 inhibitor attenuated the cortisol-induced decrease in expression of the dermal papilla biomarkers Wnt5a and ALP in DPCs. These findings suggest that blockage of 11β-HSD1 activity in DPCs can partially restore the glucocorticoid-induced impairment of dermal papilla properties. Further studies are needed to confirm whether the inhibition of 11β-HSD1 can restore the function of DPCs when exposed to glucocorticoids in vivo.
In conclusion, we have shown an inhibitory effect of glucocorticoids on the proliferation and the expression of dermal papilla biomarkers, Wnt5a and ALP, and VEGF in cultured human DPCs. We also demonstrated a functional expression of 11β-HSD1 and its regulation by cortisol in DPCs. Our data also showed that inhibition of 11β-HSD1 can partially prevent glucocorticoid-induced suppression of proliferation, growth factor expression, and the expression of dermal papilla markers in cultured DPCs. Taken together, these results provide insights into the mechanisms underlying chronic stress-related hair loss and suggest that 11β-HSD1 inhibitors can potentially be used to prevent stress-related hair loss.
Figures and Tables
Fig. 1
Expression of 11β-HSD1 in human scalp hair follicles by immunohistochemistry. 11β-HSD1 immunoreactivity was found in ORS keratinocytes (A), MK, and the DP in human scalp hair follicles (B-E) in situ. 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; ORS, outer root sheath; MK, matrix keratinocytes; DP, dermal papilla. Scale bar=50 µm.
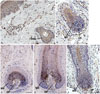
Fig. 2
Western blot analysis of 11β-HSD1 expression in unstimulated and cortisol-stimulated human DPCs. Bars show the results of densitometric analysis of the 11β-HSD1 protein band relative to the corresponding GAPDH protein band. Results are presented as mean±SD. *p<0.05. 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; DPC, dermal papilla cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.

Fig. 3
The effect of a selective 11β-HSD1 inhibitor on the proliferation of cortisol-stimulated human DPCs. (A) Human DPCs were treated with 10-7 M cortisol for 48 hours with or without 30 min pretreatment with 385581 (100 nmol L-1), and immunofluorescent staining was performed with anti-Ki-67 antibody. Nuclei were counterstained with DAPI (Scale bar, 200 µm). (B) The percentage of Ki-67-positive human DPCs (green fluorescence in nuclei) under at least five high power fields in each slide was counted, and statistical analysis was performed using a pair test. Results are presented as mean±SD. *p<0.05, †p<0.01. 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; DPC, dermal papilla cells; DAPI, 4′,6′-diamidino-2-phenylindole.

Fig. 4
The effect of a selective 11β-HSD1 inhibitor on the expression of Wnt5a, ALP, and VEGF in cortisol-stimulated human DPCs. Human DPCs were pre-treated with 100 nmol L-1 11β-HSD1 inhibitor for 30 minutes and stimulated with 10-7 M cortisol for 48 hours. (A) After stimulation, the expression of Wnt5a, ALP, and VEGF was analyzed by Western blot. (B) Bars show the results of densitometric analysis relative to the corresponding GAPDH protein band. Results are presented as mean±SD. *p<0.05, †p<0.01. 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; ALP, alkaline phosphatase; VEGF, vascular endothelial growth factor; DPC, dermal papilla cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from Yonsei University College of Medicine for 2015 (6-2015-0165).
References
1. Alexopoulos A, Chrousos GP. Stress-related skin disorders. Rev Endocr Metab Disord. 2016; 17:295–304.

2. Taheri R, Behnam B, Tousi JA, Azizzade M, Sheikhvatan MR. Triggering role of stressful life events in patients with alopecia areata. Acta Dermatovenerol Croat. 2012; 20:246–250.
3. Botchkarev VA. Stress and the hair follicle: exploring the connections. Am J Pathol. 2003; 162:709–712.
4. Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006; 15:1–13.

5. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005; 19:1332–1334.

6. Zhang X, Yu M, Yu W, Weinberg J, Shapiro J, McElwee KJ. Development of alopecia areata is associated with higher central and peripheral hypothalamic-pituitary-adrenal tone in the skin graft induced C3H/HeJ mouse model. J Invest Dermatol. 2009; 129:1527–1538.

7. Novak MA, Hamel AF, Coleman K, Lutz CK, Worlein J, Menard M, et al. Hair loss and hypothalamic-pituitary-adrenocortical axis activity in captive rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2014; 53:261–266.
8. Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, et al. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R1657–R1662.

9. Demerjian M, Choi EH, Man MQ, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009; 18:643–649.

10. Paus R, Handjiski B, Czarnetzki BM, Eichmüller S. A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol. 1994; 103:143–147.

11. Pérez P, Page A, Bravo A, Del Río M, Giménez-Conti I, Budunova I, et al. Altered skin development and impaired proliferative and inflammatory responses in transgenic mice overexpressing the glucocorticoid receptor. FASEB J. 2001; 15:2030–2032.

12. Choi SJ, Cho AR, Jo SJ, Hwang ST, Kim KH, Kwon OS. Effects of glucocorticoid on human dermal papilla cells in vitro. J Steroid Biochem Mol Biol. 2013; 135:24–29.

13. Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004; 25:831–866.

14. Tiganescu A, Walker EA, Hardy RS, Mayes AE, Stewart PM. Localization, age- and site-dependent expression, and regulation of 11β-hydroxysteroid dehydrogenase type 1 in skin. J Invest Dermatol. 2011; 131:30–36.

15. Lee SE, Kim JM, Jeong MK, Zouboulis CC, Lee SH. 11β-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. Br J Dermatol. 2013; 168:47–55.

16. Terao M, Katayama I. Local cortisol/corticosterone activation in skin physiology and pathology. J Dermatol Sci. 2016; 84:11–16.

18. Katsuoka K, Schell H, Wessel B, Hornstein OP. Effects of epidermal growth factor, fibroblast growth factor, minoxidil and hydrocortisone on growth kinetics in human hair bulb papilla cells and root sheath fibroblasts cultured in vitro. Arch Dermatol Res. 1987; 279:247–250.

19. Hembree JR, Harmon CS, Nevins TD, Eckert RL. Regulation of human dermal papilla cell production of insulin-like growth factor binding protein-3 by retinoic acid, glucocorticoids, and insulin-like growth factor-1. J Cell Physiol. 1996; 167:556–561.

20. Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001; 107:69–82.

21. Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005; 09. 20. DOI: 10.1371/journal.pbio.0030331. [Epub].

22. Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007; 49:185–195.

23. McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003; 121:1267–1275.

24. Ohyama M, Kobayashi T, Sasaki T, Shimizu A, Amagai M. Restoration of the intrinsic properties of human dermal papilla in vitro. J Cell Sci. 2012; 125(Pt 17):4114–4125.

25. Feig PU, Shah S, Hermanowski-Vosatka A, Plotkin D, Springer MS, Donahue S, et al. Effects of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor, MK-0916, in patients with type 2 diabetes mellitus and metabolic syndrome. Diabetes Obes Metab. 2011; 13:498–504.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download