Abstract
Empathy is the ability to identify with or make a vicariously experience of another person's feelings or thoughts based on memory and/or self-referential mental simulation. The default mode network in particular is related to self-referential empathy. In order to elucidate the possible neural mechanisms underlying empathy, we investigated the functional connectivity of the default mode network in subjects from a general population. Resting state functional magnetic resonance imaging data were acquired from 19 low-empathy subjects and 18 medium-empathy subjects. An independent component analysis was used to identify the default mode network, and differences in functional connectivity strength were compared between the two groups. The low-empathy group showed lower functional connectivity of the medial prefrontal cortex and anterior cingulate cortex (Brodmann areas 9 and 32) within the default mode network, compared to the medium-empathy group. The results of the present study suggest that empathy is related to functional connectivity of the medial prefrontal cortex/anterior cingulate cortex within the default mode network. Functional decreases in connectivity among low-empathy subjects may reflect an impairment of self-referential mental simulation.
Empathy is the ability to identify with or vicariously experience another person's feelings or thoughts.1 Previous studies have reported that various brain regions are involved in empathy, such as the anterior cingulate cortex (ACC), anterior insula, inferior parietal lobe, premotor cortex, posterior superior temporal sulcus, medial prefrontal cortex (mPFC), posterior cingulate cortex, precuneus, temporal pole, and temporoparietal junction.2345 However, the intrinsic interactions of these empathy-related regions has not been reported, as studies have typically been conducted using participants performing specific empathy-related tasks.6 To overcome this limitation, resting state functional neuroimaging studies of empathy should be performed.
The default mode network (DMN) consists of the ACC, mPFC, posterior cingulate cortex, inferior parietal lobule, and precuneus7 and, accordingly, overlaps with several empathy-related regions, as shown in previous task-based magnetic resonance imaging (MRI) studies.58 The DMN is thought to play a role in internally focused thought processes, including the construction of mental simulations based on previous personal experiences.910 Given that empathy is achieved by simulating the mental processes that are likely to be operating in others11 and/or by using personal memories to understand situations of other,12 the DMN is a clear candidate network underlying empathy. On this premise, we hypothesized that low resting-state functional connectivity within the DMN might exist in low-empathy participants. To address this question, we used resting-state functional MRI to evaluate DMN functional connectivity in low-versus medium-empathy individuals.
A total of 484 student participants from a local university were screened using the Korean version of the Interpersonal Reactivity Index (IRI).13 Lower IRI scores indicated a weaker degree of empathy. After all individual IRI scores were calculated, the participants with IRI scores in the 15th percentile and the 30th to 70th percentile were selected as low- or medium-empathy candidates, respectively. The medium-empathy group was chosen as a control group, because this group was more likely to represent the general population. Due to a significant sex difference in IRI scores, different cutoff scores were applied according thereto. Sex was balanced across groups in final analysis. All participants were healthy with normal cognitive function and showed no signs of psychiatric illness (as confirmed by a psychiatric interview). The exclusion criteria included a history of psychiatric or neurologic disorder, left-handedness, and the presence of an MRI-incompatible implant.
Twenty low-empathy participants and 19 medium-empathy participants were included in the study. However, the data of only 19 low-empathy participants and 18 medium-empathy participants were included in the final analysis: two participants (one from each group) were excluded due to excessive head movement during the MRI scan. Verbal IQ was assessed using the verbal scales of the Wechsler Adult Intelligence Scale. As depression and anxiety may be associated with abnormal levels of empathy1415 and abnormal DMN connectivity,1617 the Hamilton Depression Rating Scale (HAM-D)18 and Hamilton Anxiety Scale (HAM-A)19 were used to assess current symptoms of depression and anxiety. All participants provided written informed consent prior to study participation. The study protocol was approved by the Konyang University Hospital Medical Ethics Committee.
The IRI is a self-reported instrument that measures trait empathy.20 It is composed of four subscales: Perspective Taking, Fantasy, Personal Distress, and Empathic Concern. Perspective Taking measures the tendency to voluntarily think from another individual's psychological perspective. Fantasy examines the tendency to feel the emotions of people in fictional situations. Personal Distress measures self-oriented anxiety and interpersonal discomfort. Empathic Concern evaluates other-oriented feelings, including compassion and sympathy. Each subscale contains eight items with each item measured on a five-point Likert scale ranging from 0 (“Does not describe me well”) to 4 (“Describes me very well”).
During resting state scanning, participants were instructed to lie still with their eyes open. Functional MRI data were acquired with a Philips 3T scanner (Philips Intera, Philips Medical System, Best, the Netherlands) equipped with an eight-channel SENSE head coil. Resting state functional images were acquired using a gradient echo-planar imaging sequence with the following parameters: 116 volumes (348 s); repetition time (TR)=3000 ms; echo time (TE)=35 ms; 33 slices; no gap; flip angle=90°; field of view (FOV)=230 mm; voxel size=1.80×1.80×4 mm. Structural images were also acquired with the following parameters: TR=536 ms; TE=10 ms; flip angle=70°; 33 slices; voxel size 0.45×0.45×4 mm; FOV=230 mm.
Preprocessing was performed using Statistical Parametric Mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London, UK). The first five images were discarded to allow for the equilibration of longitudinal magnetization. Individual scans were realigned and slice time-corrected, normalized to a standard SPM5 template based upon the Montreal Neurological Institute (MNI) reference brain, and spatially smoothed using a 10-mm isotropic Gaussian kernel with standard SPM methods. Motion parameters for each individual were visually inspected, and only data with translation motion less than 2 mm and rotational movement less than 2° in any direction were included.
Independent component analysis (ICA) is a data-driven method to identify spatially independent components of brain areas with hemodynamic time courses that closely covary. Thus, the regions comprising each component are conceptualized as parts of a specific network with highly synchronous time courses.21 For group comparisons, a separate group ICA may not be optimal, because it is biased towards false-positive results.2122 Therefore, images of all participants were decomposed into sets of independent components using the Group ICA FMRI Toolbox (GIFT) and the Infomax algorithm.21
The group ICA was carried out in three stages: data reduction, application of the ICA algorithm, and backwards reconstruction for each participant. First, data from each participant underwent a principal component analysis to reduce computational complexity. Next, the reduced participant data were concatenated over the time domain. To determine the number of independent components, a dimensionality estimation was performed using the minimum description length criteria.23 The ICA estimation identified 18 components. In the second stage of the analysis, we used the Infomax algorithm to run the ICA and a mask based on all participants. In the final stage of backwards reconstruction, time courses and spatial maps were reconstructed and converted to Z-scores to normalize signals.24
Selection of the DMN component was completed in two stages. First, each of the 18 components identified by the group ICA was manually inspected for the presence of obvious artifacts. Then, the individual independent components were spatially sorted using a DMN mask provided within the GIFT toolbox. The mask consisted of the posterior cingulate cortex [Brodmann areas (BAs) 23 and 31], posterior parietal cortex (BAs 7, 39, and 40), dorsolateral and superior frontal cortices (BAs 8, 9, and 10), ACC (BAs 11 and 32), and inferior temporal gyrus (BAs 19 and 37). The component that showed the highest correlation with the DMN template was selected as the DMN (r=0.58).
Independent t-tests and chi-square tests were applied to test for significant group differences in demographic and clinical variables. Selected best-fit components were entered into a second level random-effects analysis in SPM5. Two sample t-tests examined group differences in the degree of regional functional connectivity. The statistical threshold for these analyses was set at p<0.001, uncorrected, with an extent threshold of 20 voxels. Anatomical regions and denominations are reported according to the atlases of Talairach and Tournoux.25 All coordinates are reported in the MNI space.
Table 1 shows the demographic and clinical data of the 19 low-empathy participants and 18 medium-empathy participants that were included in our study. There were no significant differences in age, sex, education, or verbal IQ, HAM-D, and HAM-A scores between the two groups. For the IRI, the overall scores of the medium-empathy group were significantly higher than those of the low-empathy group; moreover, the IRI subscale scores of the medium-empathy group were higher than those of the low-empathy group for all subscales, except for Perspective Taking.
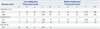
Table 2 shows the anatomical location and Talairach coordinates for the peak activation voxel in each brain region (x, y, z), as well as the t scores from our random effects analyses [p<0.05 family-wise error (FWE)-corrected, k>40 voxels]. Individuals in the low-empathy group showed a lower functional connectivity of the mPFC/ACC within the DMN (BAs 9 and 32, x=15, y=39, z=15, t=4.51, k=23 voxels, uncorrected p<0.001) than that of the medium-empathy group (Fig. 1).
The functional connectivity strength of the mPFC/ACC was positively correlated with the scores of the IRI and all IRI subscales (r=0.59, p<0.001 for IRI; r=56, p<0.001 for Empathic Concern; r=0.48, p<0.01 for Fantasy; r=0.37, p<0.05 for Personal Distress), with the exception of Perspective Taking.
The aim of the present study was to investigate resting state differences in DMN connectivity between low- and medium-empathy participants. Direct group comparisons revealed lower functional connectivity of the mPFC/ACC within the DMN of low-empathy participants. Therefore, a decreased functional connectivity of the mPFC/ACC within the DMN might underlie specific deficits in empathy.
According to the internal mentation hypothesis, the DMN consists of two distinct interacting subsystems;10 one is the medial temporal lobe subsystem, which is activated during the successful retrieval of old information from memory,2627 and the other is the mPFC subsystem, which is activated during self-referential mental simulation.2829 Buckner, et al.10 interpreted self-referential mental simulation as thinking about the complex interactions among people that are perceived as being socially, interactively, and emotively similar to those of oneself. This interpretation suggests that empathy is, at least in part, based on self-referential mental simulation and requires the ability to relate to the feelings or thoughts of others without losing sight of one's own feelings or thoughts.30 Given our results regarding mPFC connectivity and the abovementioned role of the mPFC in self-referential mental simulation, the present study suggests that low-empathy individuals may have impaired or decreased self-referential mental simulation during the resting state. In other words, low-empathy individuals may show decreased functional connectivity among regions of the DMN responsible for self-referential mental simulation, whereas connectivity is better sustained in normal medium-empathy individuals.
Previous studies have reported altered DMN connectivity in various psychiatric disorders associated with a lack of empathy, including autistic spectrum disorder,31 schizophrenia,32 and antisocial personality disorder.33 Of note, the present study identified low DMN connectivity in low-empathy participants of a general population rather than a clinical population. Consistent with this finding, a previous study reported altered functional connectivity of the DMN in general population participants with alexithymia.34 Therefore, various features of DMN connectivity may be related to individual trait differences among individuals from the general population, making the DMN a useful target for the investigation of personality traits, specifically empathy.
The limitations of our study should be noted. First, participants could have made an error in measuring the degree of their own empathy, thus the IRI, as a self-report assessment, may not have been valid. Using objective measurements for empathy could overcome this limitation. Second, the current sample size should have been larger for more valid results. Third, although we recorded the psychiatric history of the participants and psychiatrists administered the HAM-D and HAM-A, standardized psychiatric interviews were not performed to assess the occurrence of psychiatric disorders. Finally, although all participants responded to the technologist at the beginning and end of the MRI scan and although none of them indicated that they slept during the scan, attentional measurements during the scan were not performed.
In conclusion, we found that low-empathy individuals exhibit diminished functional connectivity of the mPFC/ACC within the DMN, which may reflect decreased self-referential mental simulation as an underlying cause of empathic deficits. Accordingly, the functional connectivity of the mPFC/ACC within the DMN may be a major factor underlying trait empathy.
Figures and Tables
 | Fig. 1Coordinates of a voxel exhibiting lower functional connectivity within the default mode network in the low-empathy empathy group, compared to the medium empathy group (x=15, y=39, z=15, t=4.51, k=23 voxels, uncorrected p<0.001). |
Table 1
Demographic and Clinical Characteristics
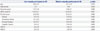
Table 2
The Anatomical Location and Talairach Coordinates for Voxels Whose Resting State Time-Course Best Fit the DMN Component (x, y, z), and t Scores from Random Effects Analyses for Low- or Medium-Empathy Group (p<0.05 FWE-Corrected, k>40 Voxels, for the Purpose of Illustration)
ACKNOWLEDGEMENTS
We would like to thank Bum Hee Park for his methodological advice.
This work was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A050495).
References
1. Deutsch F, Madle RA. Empathy: historic and current conceptualizations, measurement, and a cognitive theoretical perspective. Hum Dev. 1975; 18:267–287.

3. Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011; 54:2492–2502.

4. Zaki J, Ochsner KN. The neuroscience of empathy: progress, pitfalls and promise. Nat Neurosci. 2012; 15:675–680.

5. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009; 21:489–510.

6. Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010; 4:8.

7. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:676–682.

8. Wagner DD, Kelley WM, Heatherton TF. Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cereb Cortex. 2011; 21:2788–2796.

10. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008; 1124:1–38.
12. Gopnik A, Astington JW. Children's understanding of representational change and its relation to the understanding of false belief and the appearance-reality distinction. Child Dev. 1988; 59:26–37.

13. Kang I, Kee SW, Kim SE, Jeong BS, Hwang JH, Song JE, et al. Reliability and validity of the Korean-version of Interpersonal Reactivity Index. J Korean Neuropsychiatr Assoc. 2009; 48:352–358.
14. Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014; 76:567–574.

15. Schreiter S, Pijnenborg GH, Aan Het Rot M. Empathy in adults with clinical or subclinical depressive symptoms. J Affect Disord. 2013; 150:1–16.

16. Guo W, Liu F, Zhang J, Zhang Z, Yu L, Liu J, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLoS One. 2014; 9:e91102.

17. Andreescu C, Sheu LK, Tudorascu D, Walker S, Aizenstein H. The ages of anxiety--differences across the lifespan in the default mode network functional connectivity in generalized anxiety disorder. Int J Geriatr Psychiatry. 2014; 29:704–712.

18. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967; 6:278–296.

20. Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Personal Soc Psychol. 1983; 44:113–126.

21. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001; 14:140–151.

22. Otti A, Guendel H, Läer L, Wohlschlaeger AM, Lane RD, Decety J, et al. I know the pain you feel-how the human brain's default mode predicts our resonance to another's suffering. Neuroscience. 2010; 169:143–148.

23. Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007; 28:1251–1266.

24. Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005; 360:1001–1013.

25. Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : an approach to cerebral imaging. New York: Thieme;1988.
26. Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004; 16:1484–1492.

27. Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006; 96:3517–3531.

28. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:4259–4264.

29. Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006; 50:655–663.

30. Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004; 3:71–100.

31. Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010; 53:247–256.

32. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007; 164:450–457.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download