Abstract
Local allergic rhinitis (LAR) is a localized nasal allergic response in the absence of systemic atopy. The aim of this study was to evaluate the prevalence and clinical characteristics of LAR in Korean rhinitis patients compared to allergic rhinitis (AR) and non-allergic rhinitis (NAR). A total of 304 rhinitis patients were enrolled from November 2014 to March 2016. A skin prick test, serum total and specific immunoglobulin E, and a nasal provocation test (NPT) with house dust mite (HDM) were performed on all patients. Subjects also documented changes in rhinitis symptoms before and after NPT. Seventy-four patients with nasal hyper-reactivity and 80 patients with subclinical allergy were excluded. AR was diagnosed in 69 (46.0%) patients, NAR in 75 (50.0%) patients, and LAR to HDM in 6 (4.0%) patients. The average medication score and disease duration of each group were 14.5 points and 77.6 months in AR, 12.1 point and 51.1 months in NAR, and 17.7 point and 106.0 months in LAR, respectively. There were no significant differences in the baseline nasal symptom score of the three groups. However, after NPT with HDM, the score of rhinitis, itching, and obstructive were 4.83±1.47 vs. 1.95±2.53, 3.00±2.10 vs. 1.45±2.06, and 5.50±1.38 vs. 2.57±2.84 in LAR and NAR, respectively (p<0.05). LAR patients had longer duration of disease and tended to be older and have higher medication score than other rhinitis patients.
Rhinitis is a common upper respiratory disease characterized by rhinorrhea, itching, sneezing, and nasal obstruction. Traditionally, rhinitis can be classified as allergic rhinitis (AR) and non-allergic rhinitis (NAR) based on clinical manifestations and allergic sensitization to common allergens.1 Recent evidences showed another class of rhinitis which represents localized nasal allergic response in the absence of systemic atopy as local allergic rhinitis (LAR). LAR can be diagnosed by a positive response to the nasal provocation test (NPT) without positive skin prick test (SPT) and serum specific immunoglobulin E (sIgE). The first concept of LAR was introduced by Huggins and Brostoff2 in 1975, indicating local production of sIgE antibodies in the nasal mucosa. However, this issue has not been completely studied yet and almost all studies have focused on the pathophysiology of LAR. Thus, the prevalence and clinical features of LAR still need to be evaluated. Furthermore, there are still few studies on the prevalence of LAR in a Korean population. In this study, we evaluated the prevalence of LAR patients and investigated the clinical characteristics and severity of LAR patients compared to AR and NAR patients in Korea.
Between November 2014 and March 2016, we recruited 304 patients (146 males and 158 females, 6–78 years old) suffering from rhinitis symptoms who visited the outpatient clinic of Ajou University Hospital. Patient clinical characteristics, such as disease duration, medication score, and comorbidities of other allergic diseases, were obtained by history taking on the first clinic visit day. SPT, serum total and sIgE measurement, and NPT with sterile saline and Dermatophagoides farinae were performed on all patients. We also checked the Visual Analogue Scale (VAS) for nasal symptoms (rhinorrhea, itching, sneezing, and obstruction) before and after NPT. Ten-scale score system to each nasal symptom was used and total score was 40. The medication scores of oral anti-histamine and intranasal glucocorticoid ranged from 0 to 7 according to the number of drug use days. The medication score of systemic glucocorticoid was scored in terms of the number of days the patient took oral corticosteroids as a rescue medication: 4=1 day, 8=2–3 days, 12=4–5 days, and 16=more than 6 days per week. In the diagnose of LAR, we excluded all patients who had weak atopy to house dust mite (HDM) based on either results of SPT (Allergen/Histamine ratio ≥1) or serum sIgE (≥0.35 kU/L). NPT was conducted as previously described.34 The patients stopped taking systemic or intranasal antihistamines, corticosteroids, and vasoconstrictor prior to NPT at least 4 weeks. They were allowed to relax for 30 minutes at room temperature. Before and 15 minutes after HDM nasal challenge, they were asked to complete the VAS on nasal symptoms (rhinorrhea, itching, sneezing, and obstruction). A positive response to NPT was defined as more than 6.5 increase in total nasal symptom scores and at least 2 increase in nasal obstruction symptom after NPT.3 The protein concentration of HDM allergen extract (Allergopharma, Reinbek, Germany) was measured to 10.1 mg/mL. This allergen extract was diluted to 1:10 with saline before challenge, and 40–50 µL of the diluted allergen solution was sprayed into each nostril. Before HDM allergen challenge, patients underwent a saline challenge test to rule out nonspecific nasal hyper-reactivity. After waiting for 15 minutes to annul the effects of the saline challenge, the challenge with HDM allergen was performed. All tests were done by the same examiner. All analyses were carried out by using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±standard deviation. The clinical and demographic data were compared between the groups by using the non-parametric Mann-Whitney U test and Fisher's exact test. A p value of <0.05 was considered statistically significant. The study protocol was approved by the Institutional Review Board of Ajou University School of Medicine (AJIRB-MED-MDB-16-385).
Among total 304 rhinitis patients, 74 patients were excluded for their positive response to saline. Eighty patients who had atopy without any response to HDM NPT were classified as subclinical and also excluded from the study. The remaining subjects were classified into 3 groups according to their atopic status and the NPT results; the AR group had atopy to HDM and positive response to NPT, the NAR group had neither any atopy to HDM nor any positive response to NPT, and finally the LAR group had no atopy to HDM but positive response to NPT. Of 150 patients, 69 (46.0%) had AR, 75 (50.0%) had NAR, and 6 (4.0%) had LAR to HDM. Their clinical characteristics are summarized in Table 1. There was no significant difference in gender, or comorbid allergic disease. However, disease duration was significantly longer in the LAR group than in the NAR group (p=0.012). The LAR group also tended to be older and have higher medication score than other groups at disease diagnosis, but the difference was not statistically significant. Fig. 1 shows the results of NPT. No significant difference was found between groups in nasal symptoms at baseline and after saline NPT. However, the LAR group had significant nasal symptoms (rhinorrhea, itching, and obstruction) compared to the NAR group after NPT with HDM allergen.
As mentioned earlier, the first concept of LAR was introduced by Huggins and Brostoff2 in 1975, and the term “local allergic rhinitis” by Rondón, et al.5 in 2009. They developed the concept of LAR and established a new etiological classification of rhinitis including LAR.6 NPT to allergens is considered as the gold standard diagnostic method in LAR. However, there are still continuing debate about the concept of LAR and a standard method of NPT. Rondon defined a positive NPT response as more than 30% increase in total VAS score after NPT and more than 30% decrease in the sum of both nasal cavity volumes using rhinometry.78 However, rhinometry is often impractical and has a limited value in the diagnosis of LAR in clinical practice. Also, rhinometry results are not accurate in patients who have nasal pathology such as chronic sinusitis or septal deviation, and most of the rhinitis patients have the above comorbid conditions. For these reasons, a new and simplified diagnostic method to LAR was introduced with symptomatic VAS change after allergen NPT. New positive criteria are more than 6.5 increase in total nasal symptom score as well as at least 2 increase in nasal obstruction symptom score after allergen provocation, and these new criteria had 90.6% sensitivity and 77.4% specificity, respectively.3 Furthermore, both sensitivity and specificity of the new method are much higher than those obtained by using rhinometry (73.4% sensitivity and 58.1% specificity). In this study, therefore, we used only the VAS system for nasal symptoms in the diagnosis of LAR.
The prevalence of LAR in the present study seems to be low compared to previous Spanish results: in a prospective study of 428 patients, LAR was diagnosed in 25.7% using NPT, VAS for nasal symptoms, and rhinometry.9 Although study design and diagnostic method were not same, ethnic difference may be one of the reasons of these discrepancies. Cheng, et al.10 reported 8.2% of LAR to HDM in Chinese, and Kim and Jang11 showed 3.5% of LAR in Korean patients.
In our study, nasal symptoms in NAR patients were improved after NPT with sterile saline and even HDM allergen, whereas there was significant symptomatic aggravation in AR and LAR (Fig. 1), indicating significantly different clinical responses to allergen between LAR and NAR, although our routine laboratory test, such as SPT and sIgE, showed that both groups had same systematic results.
There are several limitations in our study. First, we used only single HDM challenge in order to save time in outpatient setting. That may explain why 80 patients were classified as subclinical allergy and our somewhat low prevalence of LAR compared to Spanish results. Standardization of allergen concentration and study protocol need to be established in future study. Secondly, in the present study, we could not measure local production of tryptase, eosinophil cationic protein, sIgE to HDM, and any interleukins. However, we previously measured sIgE and mediators according to NPT results.4 Finally, the natural course of LAR was not evaluated in our study due to a shortterm follow-up. Further prospective multi-center studies in welldefined patients with LAR are needed to confirm our present results.
In summary, the prevalence of LAR to HDM is low in Korean rhinitis population, but they have a longer duration of disease, and tend to be older and have higher medication score compared to other rhinitis patients. The results of this study suggest that LAR should be considered in patients with severe AR symptoms but no systemic allergic responses.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0992).
References
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160. PMID: 18331513.
2. Huggins KG, Brostoff J. Local production of specific IgE antibodies in allergic-rhinitis patients with negative skin tests. Lancet. 1975; 2:148–150. PMID: 49744.

3. Jang TY, Kim YH. Nasal provocation test is useful for discriminating allergic, nonallergic, and local allergic rhinitis. Am J Rhinol Allergy. 2015; 29:e100–e104. PMID: 26163237.

4. Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016; 8:329–337. PMID: 27126726.

5. Rondón C, Fernández J, López S, Campo P, Doña I, Torres MJ, et al. Nasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitis. J Allergy Clin Immunol. 2009; 124:1005–1011. PMID: 19796796.

6. Rondón C, Fernandez J, Canto G, Blanca M. Local allergic rhinitis: concept, clinical manifestations, and diagnostic approach. J Investig Allergol Clin Immunol. 2010; 20:364–371.
7. Rondón C, Romero JJ, López S, Antúnez C, Martín-Casañez E, Torres MJ, et al. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol. 2007; 119:899–905. PMID: 17337294.

8. Rondón C, Campo P, Herrera R, Blanca-Lopez N, Melendez L, Canto G, et al. Nasal allergen provocation test with multiple aeroallergens detects polysensitization in local allergic rhinitis. J Allergy Clin Immunol. 2011; 128:1192–1197. PMID: 21783237.

9. Rondón C, Campo P, Galindo L, Blanca-López N, Cassinello MS, Rodriguez-Bada JL, et al. Prevalence and clinical relevance of local allergic rhinitis. Allergy. 2012; 67:1282–1288. PMID: 22913574.

10. Cheng KJ, Xu YY, Liu HY, Wang SQ. Serum eosinophil cationic protein level in Chinese subjects with nonallergic and local allergic rhinitis and its relation to the severity of disease. Am J Rhinol Allergy. 2013; 27:8–12. PMID: 23406588.

11. Kim YH, Jang TY. Clinical characteristics and therapeutic outcomes of patients with localized mucosal allergy. Am J Rhinol Allergy. 2010; 24:e89–e92. PMID: 20819459.

Fig. 1
Change of nasal symptom patterns assessed by the Visual Analogue Scale (VAS). Data are expressed as mean±standard deviation. *p<0.05, †p<0.01 compared to each group. AR, allergic rhinitis; LAR, local allergic rhinitis; NAR, non-allergic rhinitis; Baseline, nasal symptoms at baseline; Saline NPT, nasal symptoms after NPT with saline; HDM NPT, nasal symptoms after NPT with Dermatophagoides farinae; NPT, nasal provocation test.
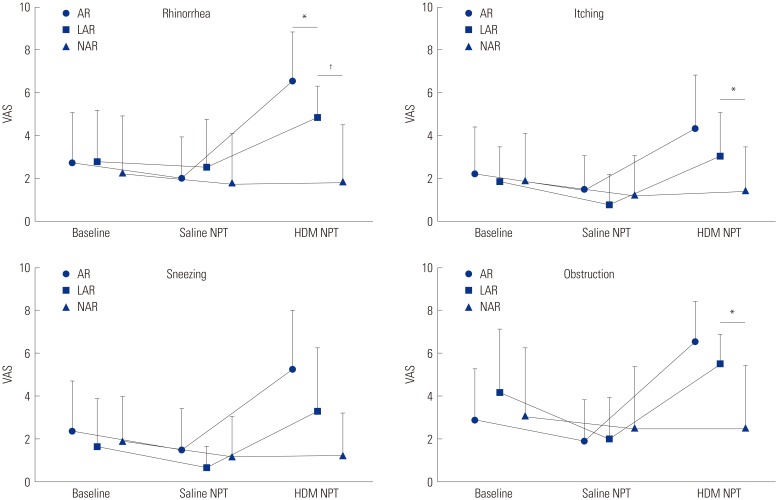
Table 1
Clinical Characteristics According to the Rhinitis Type
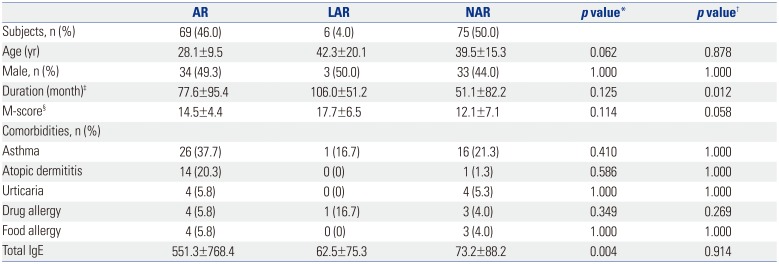
AR, allergic rhinitis; LAR, local allergic rhinitis; NAR, non-allergic rhinitis; IgE, immunoglobulin E.
Continuous variables are expressed as mean±standard deviation.
*Significant difference between LAR and AR, †Significant difference between LAR and NAR, ‡Disease in duration, §Medication score at the first clinic visit.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download