Abstract
Purpose
Most studies on immune tolerance of mesenchymal stem cells (MSCs) have been performed using MSCs derived from bone marrow, cord blood, or adipose tissue. MSCs also exist in the craniofacial area, specifically in teeth. The purpose of this study was to evaluate the mechanisms of immune tolerance of dental pulp-derived MSC (DP-MSC) in vitro and in vivo.
Materials and Methods
We isolated DP-MSCs from human dental pulp and co-cultured them with CD4+ T-cells. To evaluate the role of cytokines, we blocked TGF-β and IL-10, separately and together, in co-cultured DP-MSCs and CD4+ T-cells. We analyzed CD25 and FoxP3 to identify regulatory T-cells (Tregs) by fluorescence-activated cell sorting (FACS) and real-time PCR. We performed alloskin grafts with and without DP-MSC injection in mice. We performed mixed lymphocyte reactions (MLRs) to check immune tolerance.
Results
Co-culture of CD4+ T-cells with DP-MSCs increased the number of CD4+CD25+FoxP3+ Tregs (p<0.01). TGF-β or/and IL-10 blocking suppressed Treg induction in co-cultured cells (p<0.05). TGF-β1 mRNA levels were higher in co-cultured DP-MSCs and in co-cultured CD4+ T-cells than in the respective monocultured cells. However, IL-10 mRNA levels were not different. There was no difference in alloskin graft survival rate and area between the DP-MSC injection group and the non-injection group. Nonetheless, MLR was reduced in the DP-MSC injected group (p<0.05).
Studies of diverse kinds of mesenchymal stem cells (MSCs) have recently been extended to include MSCs from many different types of tissues. Although teeth are hard tissues, they contain stem cells originating from dental pulp, periodontal ligaments, apical papilla, and exfoliated deciduous teeth.123
MSCs modulate inflammatory reactions and suppress T-cell proliferation.4 One of the mechanisms of immune modulation by MSCs is the induction of regulatory T-cells (Tregs).568 In addition, soluble factors secreted from MSCs increase Tregs.7 Early studies of MSCs with diverse functions were performed mainly using MSCs derived from bone marrow (BM-MSCs).567 Because many kinds of MSCs have been found in different sources, MSC studies have expanded to study MSC characteristics depending on the specific source of the MSCs.91011
Tolerance studies have indicated increased importance not only for solid organ transplantation but also for vascularized composite allotransplantation (VCA). Based on the concept of “like with like,” the numbers of hand transplantations and facial transplantations have been increasing, and satisfactory results have been reported.1213 Immunosuppressive agents should be used throughout a patient's life after VCA; however, side effects can include infection, malignancy, and diabetes. Therefore, studies of the immune tolerance of MSCs associated with VCA have been performed to reduce the requirement for immunosuppressants.14
MSCs of dental origin are known to modulate immune resp-onses,315161718 although it is not yet known whether stem cells of various dental origins have the same mechanisms of immune tolerance as other stem cells. We investigated the mechanism of immune tolerance by dental pulp-derived MSC (DP-MSC). We analyzed CD4+CD25+FoxP3+ Tregs to evaluate the effect of DP-MSCs on immune tolerance. We also investigated the effects of the soluble factors TGF-β and IL-10, and we examined the immune tolerance effects in vivo.
We obtained normal human premolars extracted for orthodontic purposes and isolated DP-MSCs from the extracted teeth according to a previously published method.19202122 The process was performed with the approval of our Institutional Review Board (Yonsei University, Gangnam Severance Hospital, IRB# 3-2012-0082). We obtained teeth from a total of 6 patients. We cleaned the surface of the teeth with sterile normal saline. We then made a crack on the teeth by dental fissure burr in the vertical direction and cut the teeth using a pin cutter. After that, we extracted the dental pulp. We cut the pulp tissues to a size of 2×2×1 mm and cultured the pulp cells to produce outgrowths from the fragments.19,21
We analyzed the membrane-associated molecules of the human DP-MSCs by fluorescence activated cell sorting (FACS). We analyzed CD13 (anti-human PE, eBioscience, San Diego, CA, USA), CD44 (anti-human Alexa Fluor 488, Molecular Probes, Rockford, IL, USA), CD73 (anti-human Alexa Fluor 488, Molecular Probes), CD90 (anti-human APC, Molecular Probes), CD105 (anti-human Alexa Fluor 488, Molecular Probes), CD31 (anti-human PE, BD Pharmingen, San Jose, CA, USA), CD34 (anti-human APC, Molecular Probes), and CD45 (anti-human PreCP-Cy5-5-A, Molecular Probes).
We isolated human peripheral blood mononuclear cells (PB-MCs) from a healthy individual by density gradient centrifugation using the Ficoll-Hypaque. We isolated CD4+ T-cells from the PBMCs using CD4+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany).7 We first counted the PBMCs and resuspended 1.0× 107 cells in 80 µL of MACS buffer [0.5% bovine serum albumin in phosphate-buffered saline (PBS)]. We then added 20 µL of CD4 microbeads (Miltenyi Biotec) to the aspirated cells. We incubated the cell suspension for 15 min at 4℃ and then applied the prepared cells to an MS column (Miltenyi Biotec) in the magnetic field of the MACS separator. We collected the labeled cells on the tube after removing the column from the separator.
We seeded 1.0×105 DP-MSCs per well on a six-well culture plate. We then added 3.0×105 CD4+ T-cells per well and incubated the cultures for 72 h at 37℃. To determine the effects of TGF-β and IL-10 on immune tolerance of the DP-MSCs, we added anti-TGF-β (10 µg/mL; R&D systems, Minneapolis, MN, USA) and/or anti-IL-10 (0.05 µg/mL; R&D systems) to the co-cultured DP-MSCs and CD4+ T-cells. The co-culture groups with blocking were TGF-β single block, IL-10 single block, and TGF-β and IL-10 double block. All of the cultures were grown for 72 h for FACS and for 24 h for real-time PCR.
To identify Tregs, we labeled the CD4+ T-cells cultured for 72 h for surface CD4 and CD25 and for intracellular FoxP3. We used CD4-FITC (anti-human CD4, Miltenyi Biotec), CD25-PE (anti-human CD25, BD Pharmingen) antibodies, and FoxP3-APC (anti-human Foxp3, eBioscience). The final cell concentration for FACS was 1.0×105 cells/mL. We performed FACS using a BD FACS Canto II Flow Cytometer and the FACSDiva software (BD Biosciences, Piscataway, NJ, USA).
After culturing them for 24 h, we analyzed CD4+ T-cells for CD25 and FoxP3. We performed semi-quantitative PCR to analyze levels of CD25, FoxP3, IL-10, and TGF-β1, 2, and 3. We investigated the cytokine origin and variation according to the cells, CD4+ T-cells or DP-MSC. We performed different experiments: DP-MSC monoculture, DP-MSC co-culture with CD4+ T-cells, CD4+ T-cell monoculture, and CD4+ T-cell co-culture with DP-MSCs. Because we did not observe any difference between mo-noculture and co-culture in TGF-β2, 3 levels, we performed real-time PCR in CD25, FoxP3, IL-10, and TGF-β1.
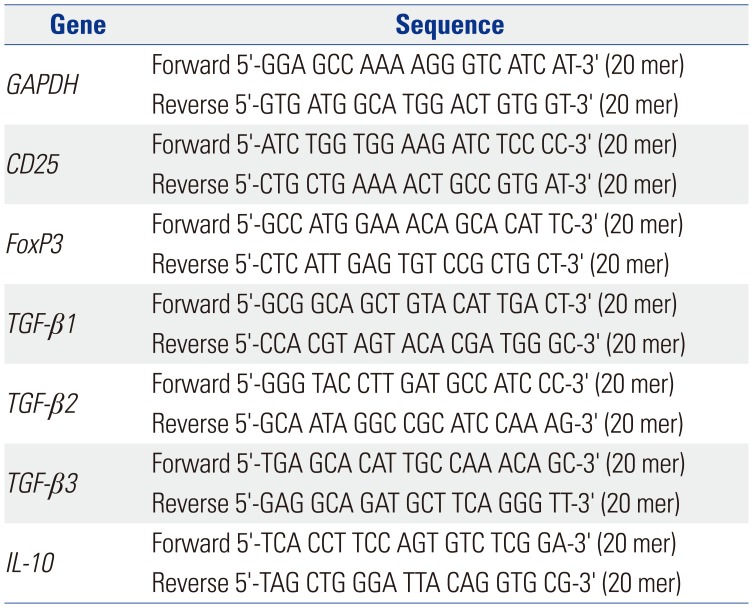
We isolated total RNA from the co-cultured CD4+ T-cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The primer sequences are summarized in Table 1. We analyzed the real-time PCR using the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). We checked the target amplification using dissociation curves. We analyzed the data using the competitive method (2^-ΔΔCt).
We performed full-thickness alloskin grafts (3×3 cm) from C57BL/6(H-2b) mice to Balb/c(H-2d) mice. The experimental group received intraperitoneal injection of 0.2 mL PBS with 1.0×106 DP-MSCs along with the graft. The control group received intraperitoneal injection of 0.2 mL PBS without DP-MSCs. We checked the status of the mice and took pictures of the grafts daily for 2 weeks. We harvested the spleens of the mice after 14 days. We calculated the size change of the grafts using IMT iSolution Lite (ver. 21.1, serial No. 818967021, IMT i-Solution, Burlington, ON, Canada).
We performed mixed lymphocyte reactions (MLRs) using the harvested spleen tissues. We compared the DP-MSC injection group with the non-injection group on postoperative day 14. We isolated responder cells using a naive CD4+ isolation kit (Miltenyi Biotec) and labeled them with 5 µM CFSE (Molecular probe, CFDA SE, Eugene, OR, USA). We seeded 2.0×105 responder cells per well in a 96-well round form plate. We treated stimulator cells with 50 µg/mL of mitomycin C (Sigma, St. Louis, MO, USA) and incubated them for 20 min at 37℃ in the dark. We then washed the cell suspension with complete media three times. We then seeded 2.0×105 treated stimulator cells per well in the 96-well round form plate and incubated the mixed lymphocyte culture for 4 days.
We used paired t-tests to analyze CD25 and FoxP3 in the co-culture experiments. We used Mann-Whitney U tests to analyze the results of the real-time PCR and MLRs. We used two-sample t-tests to analyze the alloskin graft survival areas. We analyzed the survival rate by the Kaplan-Meier method. Statistical analyses were conducted using SPSS version 23 (IBM SPSS Statistics, Armonk, NY, USA). Statistical significance was set at p<0.05.
After re-culturing the cells for three-five passages, we checked the positive and negative MSC markers. CD13, CD44, CD73, CD90, and CD105 were expressed as positive markers. CD31, CD34, and CD45 were not expressed (Fig. 1).
FACS showed that the number of CD4+CD25+FoxP3+ Tregs was higher in the co-cultures of CD4+ T-cells and DP-MSCs than in the monocultures of CD4+ T-cells (p=0.003, paired t-test). Relative to that in the co-cultures without cytokine blocking, the number of Tregs was decreased in the co-cultures with single blocking of TGF-β or IL-10 (p=0.039 and 0.005, respectively, paired t-test). The decrease in the number of CD4+CD25+FoxP3+ Tregs in the co-cultures with double blocking was greater than that in the co-cultures with single blocking (p=0.001, paired t-test); however, the difference was not cumulative (Fig. 2).
After semi-quantitative PCR, the CD25 and FoxP3 bands from the CD4+ T-cells co-cultured with DP-MSCs were more intense than those from the monocultured CD4+ T-cells (Fig. 3). Real-time PCR showed that the CD25 and FoxP3 mRNA levels in the CD4+ T-cells co-cultured with DP-MSCs were higher than those in the monocultured CD4+ T-cells (CD25, p=0.001; FoxP3, p=0.001; Mann-Whitney U tests) (Fig. 4). Likewise, the TGF-β1 mRNA levels in the CD4+ T-cells co-cultured with DP-MSCs and in the DP-MSC co-cultured with CD4+ T-cells were higher than those in the respective monocultured cells (p=0.002; p=0.007; Mann-Whitney U tests). Although the IL-10 mRNA levels were higher in the co-cultured cells relative to those in the respective monocultured cells, the differences were not statistically significant.
We analyzed the survival area and the survival rate of alloskin grafts. Although the DP-MSC injection group showed an increased survival area, the difference was not statistically significant (Fig. 5). There was no statistical difference in survival rate.
We performed MLR analysis of splenocytes from Balb/c mice that received alloskin grafts with or without DP-MSCs. The responder cells were the splenocytes of the mice that received the grafts. The stimulator cells were the splenocytes of C57BL/6 mice. We analyzed CD4+ splenocytes using CD4-APC and CFSE staining. The mice that received DP-MSCs showed decreased lymphocyte reaction, compared with the mice that did not receive DP-MSCs (p=0.04, Mann- Whitney U test) (Fig. 6).
Early studies of MSCs mostly used BM-MSCs.46 As the sources of MSCs were extended, research on MSCs was extended to stem cells derived from cord blood and other sources.9 MSCs from teeth are one kind of MSC from the craniofacial area.123 MSCs of dental origin show similar MSC characteristics in terms of differentiation, multipleutic potential, and CD markers.2324
Shortly after research on MSCs began, MSCs were found to reduce inflammation.4925 Impacting immune tolerance, MSCs have been shown to affect T-cells, B-cells, and dendritic cells.26 In addition, it has been reported that the mechanisms of MSC immune modulation involve cell-to-cell contact and soluble factors.717 Immunological tolerance has mainly been addressed in the fields of immunology and transplantation. There has been an increasing amount of research on immune tolerance in plastic surgery as the success of VCA has increased. In particular, the use of MSCs has been reported to increase immunotolerance in allotransplantation.1427
Characteristics of dental-origin stem cells have also been reported in terms of regeneration and inhibition of inflammation.2428 There have been several studies on the immune tolerance and reduction of inflammation by dental-origin stem cells.315161718 Although there are various types of dental stem cells and mechanisms of immune tolerance, all the processes and mechanisms have not yet been elucidated. Dental-origin stem cells differ in biological characteristics, such as colony growth rate, proliferation, and differentiation, depending on their origin.24
Although DP-MSCs have been shown to increase Tregs in vivo in order to generate immune tolerance, few studies of cytokines have been performed in vitro. We investigated the effects of DP-MSCs on Treg variation. We used a blocking method to determine which cytokines affect the Treg variation. We also investigated the effects of DP-MSCs on immune tolerance in an alloskin graft mouse model.
In our study, CD4+CD25+FoxP3+ Treg was increased when CD4+ T-cells were co-cultured with DP-MSC rather than monocultured. Semi-quantitative PCR and real-time PCR results confirmed a significant increase in CD25 and FoxP3 mRNAs. To determine the interplay factors, we investigated mRNA of TGF-β1 and IL-10, and we added cytokine blocks experiments in the co-cultures.
We designed 3×3 cm alloskin grafts. Some previous studies designed 1×1 cm alloskin grafts. We decided that a small-sized wound could not reflect the pure alloskin graft effect. We also intended to expose the recipient mice to more antigen. We analyzed alloskin grafts using two methods: survival rate and survival area. The actual alloskin graft was not completely necrotic at a certain time point. Therefore, we measured the alloskin graft survival area.
Our results suggest that DP-MSCs increase the expression of CD4+CD25+FoxP3+ Tregs in vitro and that TGF-β1 and IL-10 are involved in the mechanism. CD4+CD25+FoxP3+ Tregs were more suppressed in the double-block assays, although the num-bers of CD4+CD25+FoxP3+ Tregs were still higher than those in the CD4+ T-cell monocultures. Those results suggest that other factors are also involved. The levels of TGF-β1 mRNA significantly increased in CD4+ T-cells co-cultured with DP-MSCs and in DP-MSCs co-cultured with CD4+ T-cells. We thought that the DP-MSCs were also affected by the CD4+ T-cells, suggesting that there was an interaction effect between the two cell types. That is somewhat different from previous reports in which cytokines secreted from MSCs affected target T-cells.8,29
Wada, et al.16 reported that MSCs require stimulating factors from activated PBMCs to exert their effects. Recently, the survival of MSCs was shown to be affected by a soluble factor from activated lymphocytes.30 We did not confirm which kind of cell elicited an effect at first or whether paracrine or autocrine effects had a greater impact. Although the difference in IL-10 mRNA was not statistically significant, CD4+CD25+ FoxP3+ Tregs were suppressed to levels similar to those caused by the TGF-β block. That result might be due to the higher potency of IL-10, even in small amounts.
We investigated the protein production of CD25, FoxP3, TGF-β1, and IL-10 by ELISA. We also performed immunohistochemical staining to observe Treg migration, TGF-β1, and IL-10 in the alloskin graft area. However, none of those factors showed any meaningful difference (results not shown).
Although the DP-MSCs had a meaningful effect on the MLR, they did not increase the survival rate or the survival area. We carefully considered that DP-MSC injection alone might not be enough to induce immune tolerance to inhibit the rejection of the alloskin grafts (Fig. 5). Many papers have suggested that MSC had immune modulation effect, and it was proved to extended survival rate or decreased rejection.27313233 MSC treatment designs on in vivo studies on transplantation or skin graft model were different according to the dose, timing, immunosuppression, or radiation before transplantation.3233 Whole-body irradiation or immunosuppressants could be together used with DP-MSC injection; however, we wanted to observe the pure effect of the DP-MSCs in the allografts. We selected intraperitoneal injection as the cell delivery method, expecting a paracrine effect. We used 3×3 cm alloskin grafts. The purpose of using the large skin grafts, compared to mouse surface areas, was to prevent bias of spontaneous wound healing in grafts of smaller size (1×1 cm) and potential exposure to antigens. Although DP-MSC injection mice had a meaningful MLR, it might not be enough to prevent against rejection, compared to control groups. Sbano, et al.34 suggested that MSC treatment combined with immunosuppression therapy would be more effective than single MSC treatment.
We found that DP-MSCs increased CD4+CD25+FoxP3+ Treg levels and were involved in immune tolerance through the reduction of the MLR. We also found that the DP-MSCs were affected by the surrounding cells and increased TGF-β1. However, the DP-MSCs did not cause a significant increase in survival rate related with immune tolerance in the actual alloskin graft. That does not mean, however, that DP-MSCs have lower potency than other MSCs. We need further study to compare DP-MSCs with other kinds of MSCs in the same conditions. In the future, it will be important to learn how MSCs interact with target cells and to understand roles played by MSCs and target cells. In addition, MSC therapy alone may not be effective as a practical application to generate immune tolerance. Further study of the cell injection amount, timing, injection method, and buster of MSCs will be needed in the future.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2012(6-2012-0051) and by funding for Young Investigator research (2014), Korean Society for Transplantation.
This study was performed based on previous adipose-derived mesenchymal stem cell research supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning(2012R1A1A1010117).
We appreciate Prof. Chooryung J. CHUNG, DDS, PhD for providing DP-MSCs and discussion. We also appreciate Dr. Tae Jo, KANG for discussion.
References
1. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000; 97:13625–13630. PMID: 11087820.
2. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002; 81:531–535. PMID: 12147742.

3. Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010; 1:5. PMID: 20504286.

4. Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003; 5:485–489. PMID: 14660044.

5. Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008; 26:212–222. PMID: 17932417.

6. Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008; 36:309–318. PMID: 18279718.
7. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009; 156:149–160. PMID: 19210524.
8. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8.

9. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301. PMID: 16410387.

10. Tark KC, Hong JW, Kim YS, Hahn SB, Lee WJ, Lew DH. Effects of human cord blood mesenchymal stem cells on cutaneous wound healing in leprdb mice. Ann Plast Surg. 2010; 65:565–572. PMID: 20948411.
11. Lequeux C, Rodriguez J, Boucher F, Rouyer O, Damour O, Mojallal A, et al. In vitro and in vivo biocompatibility, bioavailability and tolerance of an injectable vehicle for adipose-derived stem/stromal cells for plastic surgery indications. J Plast Reconstr Aesthet Surg. 2015; 68:1491–1497. PMID: 26282247.
12. Khalifian S, Brazio PS, Mohan R, Shaffer C, Brandacher G, Barth RN, et al. Facial transplantation: the first 9 years. Lancet. 2014; 384:2153–2163. PMID: 24783986.

13. Sarhane KA, Tuffaha SH, Broyles JM, Ibrahim AE, Khalifian S, Baltodano P, et al. A critical analysis of rejection in vascularized composite allotransplantation: clinical, cellular and molecular aspects, current challenges, and novel concepts. Front Immunol. 2013; 4:406. PMID: 24324470.

14. Plock JA, Schnider JT, Zhang W, Schweizer R, Tsuji W, Kostereva N, et al. Adipose- and bone marrow-derived mesenchymal stem cells prolong graft survival in vascularized composite allotransplantation. Transplantation. 2015; 99:1765–1773. PMID: 26102613.

15. Ding G, Liu Y, An Y, Zhang C, Shi S, Wang W, et al. Suppression of T cell proliferation by root apical papilla stem cells in vitro. Cells Tissues Organs. 2010; 191:357–364. PMID: 20090301.

16. Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009; 219:667–676. PMID: 19160415.

17. Zhao Y, Wang L, Jin Y, Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res. 2012; 91:948–954. PMID: 22904205.

18. Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005; 80:836–842. PMID: 16210973.

19. Yang H, Shin S, Ahn J, Choi Y, Kim KH, Chung CJ. Local injection of pulp cells enhances wound healing during the initial proliferative phase through the stimulation of host angiogenesis. J Endod. 2013; 39:788–794. PMID: 23683280.
20. Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006; 324:225–236. PMID: 16440193.

21. Park SH, Hsiao GY, Huang GT. Role of substance P and calcitonin gene-related peptide in the regulation of interleukin-8 and monocyte chemotactic protein-1 expression in human dental pulp. Int Endod J. 2004; 37:185–192. PMID: 15009408.

22. Chung CR, Kim HN, Park Y, Kim MJ, Oh YJ, Shin SJ, et al. Morphological evaluation during in vitro chondrogenesis of dental pulp stromal cells. Restor Dent Endod. 2012; 37:34–40.
23. Takeda T, Tezuka Y, Horiuchi M, Hosono K, Iida K, Hatakeyama D, et al. Characterization of dental pulp stem cells of human tooth germs. J Dent Res. 2008; 87:676–681. PMID: 18573990.

24. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806. PMID: 19767575.
25. Ichim TE, Harman RJ, Min WP, Minev B, Solano F, Rodriguez JP, et al. Autologous stromal vascular fraction cells: a tool for facilitating tolerance in rheumatic disease. Cell Immunol. 2010; 264:7–17. PMID: 20537320.

26. Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016; 8:268–278. PMID: 27679683.

27. Jeong SH, Ji YH, Yoon ES. Immunosuppressive activity of adipose tissue-derived mesenchymal stem cells in a rat model of hind limb allotransplantation. Transplant Proc. 2014; 46:1606–1614. PMID: 24935335.

28. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007; 13:151–157. PMID: 17305615.

29. Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012; 91:1003–1010. PMID: 22988011.
30. Valencic E, Loganes C, Cesana S, Piscianz E, Gaipa G, Biagi E, et al. Inhibition of mesenchymal stromal cells by pre-activated lymphocytes and their culture media. Stem Cell Res Ther. 2014; 5:3. PMID: 24405828.

31. Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res. 2014; 188:280–289. PMID: 24560349.

32. Casiraghi F, Perico N, Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant. 2013; 18:51–58. PMID: 23254705.

33. Heyes R, Iarocci A, Tchoukalova Y, Lott DG. Immunomodulatory role of mesenchymal stem cell therapy in vascularized composite allotransplantation. J Transplant. 2016; 2016:6951693. PMID: 27822384.

34. Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I, et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res. 2008; 300:115–124. PMID: 18259766.

Fig. 1
DP-MSC surface marker analysis using flow cytometry. (A) CD13 positive, (B) CD44 positive, (C) CD73 positive, (D) CD90 positive, and (E) CD105 positive. (F) CD31 negative, (G) CD34 negative, and (H) CD45 negative. These signals are shown as blue lines, while isotype matched control antibodies are shown as red lines. DP-MSC, dental pulp-derived mesenchymal stem cell.
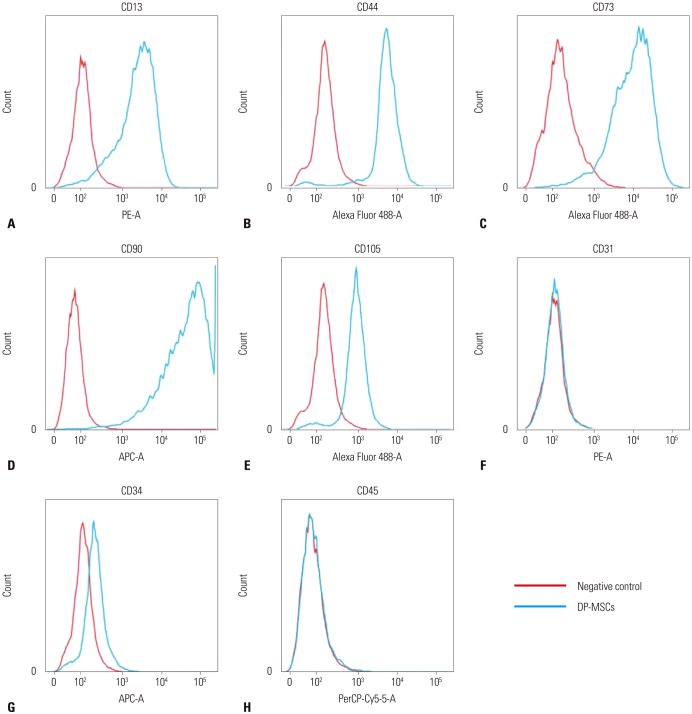
Fig. 2
Changes in CD4+CD25+FoxP3+ Tregs upon DP-MSC co-culture with and without cytokine blocking. Statistical differences were assessed by paired t tests (n=9). CD4+CD25+FoxP3+ Tregs increased in the co-cultured group (p=0.003) and decreased in the double-block group (p<0.01). The single-block groups also showed statistical differences (p<0.05). *p<0.05, †p<0.01. DP-MSC, dental pulp-derived mesenchymal stem cell; Tregs, regulatory T-cells.
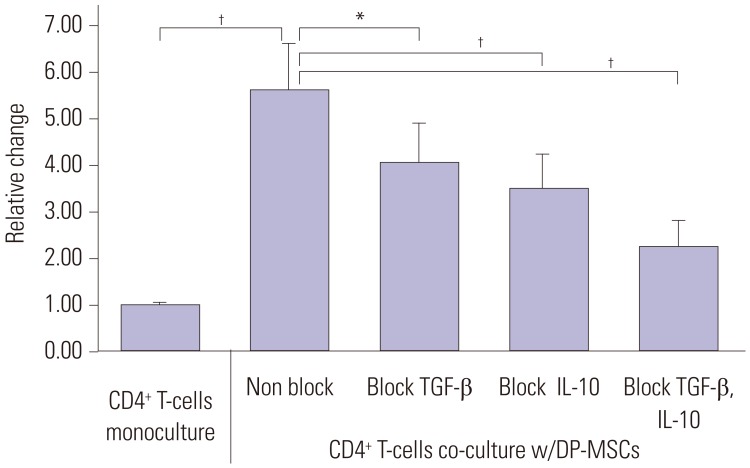
Fig. 3
Semi-quantitative PCR of CD25 (A) and FoxP3 (B). DP-MSC, dental pulp-derived mesenchymal stem cell.
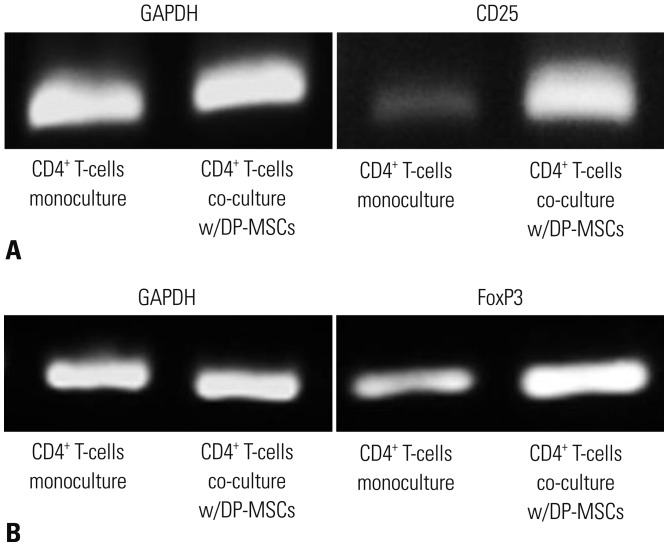
Fig. 4
Real-time PCR of monocultured CD4+ T-cells and CD4+ T-cells co-cultured with DP-MSCs. (A and B) CD25 and FoxP3 expression was higher in the co-cultured CD4+ T-cells (p=0.001, Mann-Whitney U test). (C and D) TGF-β1 expression was higher in DP-MSCs co-cultured with CD4+ T-cells than in monocultured DP-MSCs (p=0.002, Mann-Whitney U test). There was no statistical difference in IL-10 expression. (E and F) TGF-β1 expression was higher in CD4+ T-cells co-cultured with DP-MSCs than in monocultured CD4+ T-cells (p=0.007, Mann-Whitney U test). Although IL-10 expression was higher in the co-cultured cells, the difference was not statistically significant. *p<0.01. DP-MSC, dental pulp-derived mesenchymal stem cell.
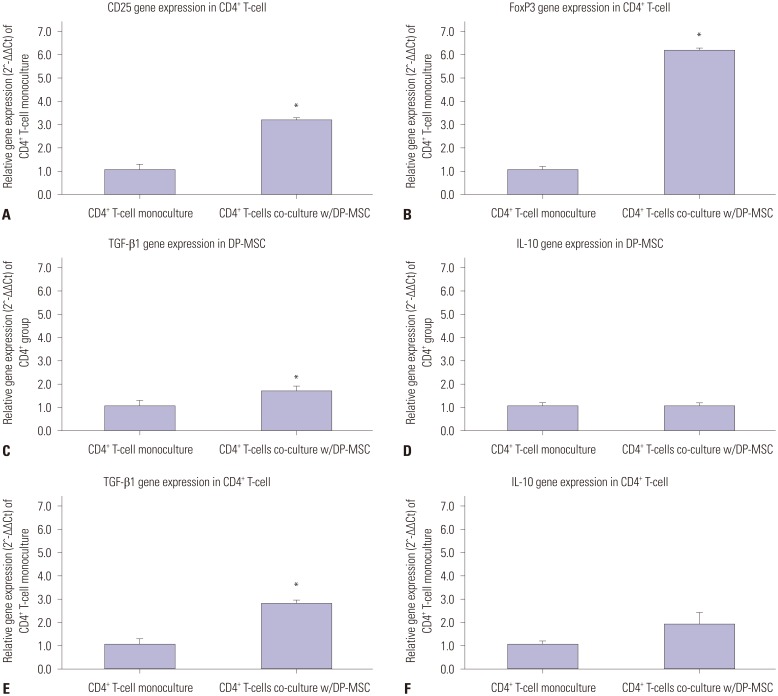
Fig. 5
Skin survival rate and area. Although the DP-MSC injection group showed elevated data, there were no statistical differences. DP-MSC, dental pulp-derived mesenchymal stem cell.
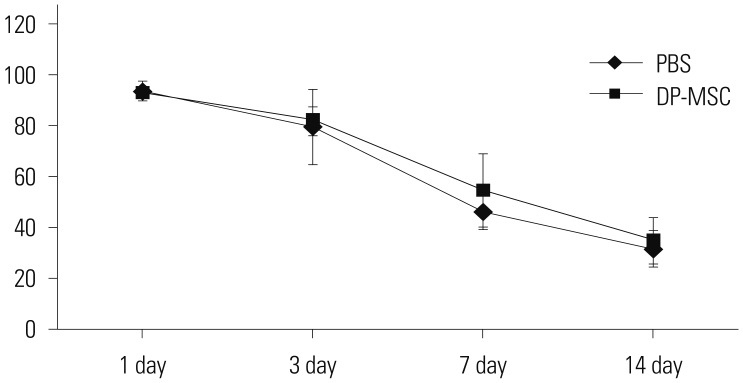
Fig. 6
MLR analysis. The PBS-injection group showed a greater increase than the DP-MSC-injection group. (A) Responder cell incubation in PBS injection group. (B) MLR with stimulator cells in the PBS injection group. (C) Responder cell incubation in DP-MSC injection group. (D) MLR with stimulator cells in DP-MSC group. (E) The CFSE-labeled cells were analyzed. The number of labeled cells of the DP-MSC-injection group was smaller than that of the PBS-injection group. There were statistical differences (p=0.04, Mann-Whitney U test). *p<0.05. DP-MSC, dental pulp-derived mesenchymal stem cell; MLR, mixed lymphocyte reaction.
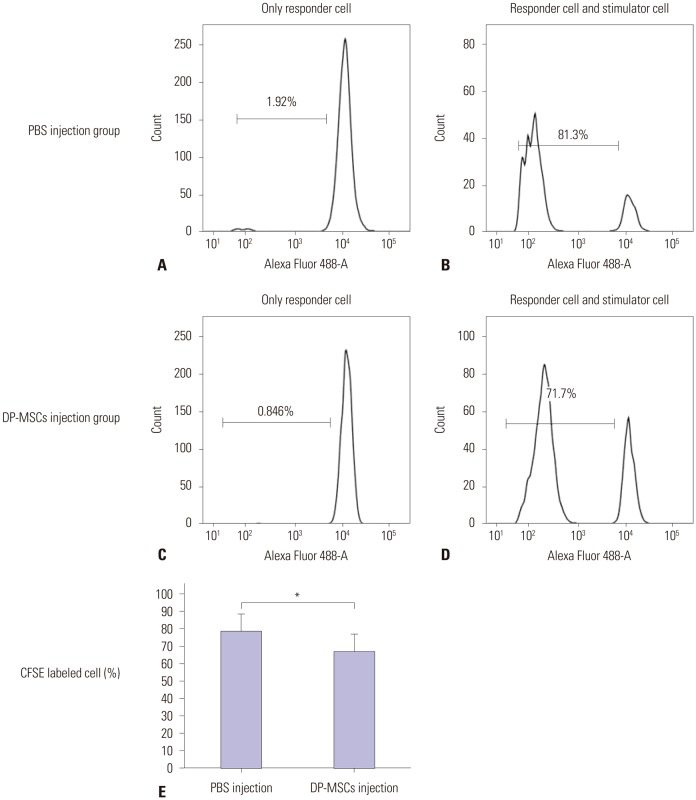
Table 1
List of Primer Pairs




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download