INTRODUCTION
Laparoscopic greater curvature plication (LGCP) is a relatively new bariatric surgery procedure. Its advantages include no stapling or rerouting of the GI tract, minimal fistula formation, no requirement for foreign body implantation, such as a gastric band, and potential reversibility. The disadvantages of LGCP are technical difficulty and limited long-term follow-up data, especially with respect to weight regain. LGCP has been compared with laparoscopic sleeve gastrectomy (LSG), because both procedures are relatively new and potentially have the same restrictive effects by reducing gastric volume, thereby producing similar anatomic changes and patterns of weight loss. LSG has rapidly gained popularity worldwide and, at present, accounts for around 27.8% of the global total number of metabolic/bariatric surgeries.
1 In a recent study by the American College of Surgeons' National Surgical Quality Improvement Program (NSQIP), Roux-en-Y gastric bypass (RYGBP), adjustable gastric band, and SG comprised 58.4, 28.8, and 9.3% of the procedures in 2010, which changed to 37.6, 3.1, and 58.2% in 2014, respectively.
2 Thus, we considered it would be worth-while to compare the effects of LGCP and LSG, as the latter has become the operative standard in terms of weight loss and resolution of comorbidities and complications. Previous comparisons of the effects of these two procedures have consistently suggested LGCP is inferior to LSG in terms of weight loss
3456 and the rate of complications requiring revision surgery.
57 On the other hand, LGCP has shown more sustained weight loss and higher levels of satisfaction Class I or II obese patients.
891011 In another study, similar positive findings were reported for this patient population for LSG.
12 In the present study, we evaluated the treatment outcomes of LGCP and LSG in obese patients with a body mass index (BMI) ranging from 30 to 35 kg/m
2.
Go to :

MATERIALS AND METHODS
This single center, retrospective study was conducted by reviewing prospectively collected data of patients that underwent either LGCP or LSG at the Gil Medical Center (Gachon University, Incheon, South Korea) from March 2013 to February 2016. These dates were selected in order to recruit patients within 3 years of surgery, that is, with a follow-up of at least 1 year. All study procedures were in accord with the ethical standards of the institutional committee at our institution (GDIRB 2013-281) and with the 1964 Declaration of Helsinki and its later amendments. The guidelines issued by the Asian Consensus Meeting on Metabolic Surgery (ACMOM 2008, Trivandrum, India) for BMI restriction using bariatric surgery (
http://www.acmoms.com/acmom_2008.html) were followed throughout. Informed consent was obtained from all study subjects, who were specifically informed that LGCP included an experimental procedure.
We have described the surgical techniques adopted for LGCP in previous studies.
1011 In brief, after gastrolysis of the greater omentum from the greater curvature of the stomach, a Bougie (36 Fr) was inserted by an anesthesiologist to guide the infolding procedure. Gastric infolding was performed using two layers of non-absorbable sutures [inner interrupted sutures of 2-0 Ethibond® (Ethicon, Somerville, NJ, USA) and outer continuous sutures of 2-0 polypropylene (Prolene®; Ethicon, Somerville, NJ, USA) or V-Loc™ (Covidien, Norwalk, CT, USA)]. The first row of sutures was started 2 cm below the gastroesophageal junction and continued until 3–4 cm proximal to the pylorus. For LSG, after gastrolysis of the greater omentum from the greater curvature, a Bougie (36 or 40 Fr) was inserted to guide gastric resection, which was performed using five to seven 60 mm staples. A serosal reinforcement suture was placed using 2-0 Vicryl® (Ethicon, Somerville, NJ, USA). Fibrin glue and a JP drain were routinely used. Patients in both groups were recommended to visit at 1, 3, 6, 12, 18, and 24 months postoperatively and then annually thereafter. Ideal body weight was defined as weight corresponding to a BMI of 23 kg/m
2 (the upper limit of normal BMI for Asian populations). Information on patient perioperative BMIs, percent excess weight loss (%EWLs), and complications were collected during follow-up outpatient visits or by e-mail or telephone. The primary study endpoints were operation time, length of hospital stay, mortality, and immediate and long-term postoperative complications requiring readmission. Secondary endpoints included weight loss (%EWL) at 3, 6, 12, 24, and 36 months postoperatively and resolution of comorbidities. Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows version 15.0 (SPSS Inc., Chicago, IL, USA). All reported
p-values are two tailed, and significance was accepted for values of <0.05.
Go to :

RESULTS
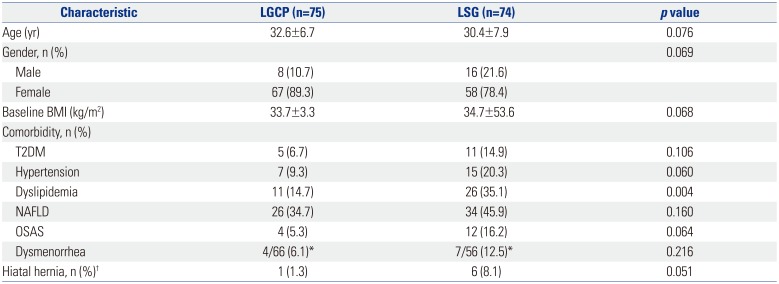
All operations were performed by a single laparoscopic surgeon (S.M.K.) and were completed laparoscopically. 149 patients were eligible, and these constituted the study cohort. The 149 study subjects were allocated to two groups, that is, 75 to group A (LGCP) and 74 to group B (LSG). Mean (±SD) ages in groups A and B were 32.6±6.7 and 30.4±7.9 years, respectively, and the number (percentages) of women were 67 (89.3%) and 58 (78.4%), respectively. Mean baseline BMIs were 33.7±3.3 and 34.7±53.6 kg/m
2, respectively. Percentages of patient with a comorbidity were not significantly different, with the exception of dyslipidemia [11 (14.7%) and 26 (35.1%), respectively,
p=0.004]. However, percentages with hiatal hernia (as documented by preoperative endoscopy) were significantly different (1.3% and 8.1%, respectively,
p=0.051) (
Table 1).
Table 1
Patient Demographic Data (n=149)
|
Characteristic |
LGCP (n=75) |
LSG (n=74) |
p value |
|
Age (yr) |
32.6±6.7 |
30.4±7.9 |
0.076 |
|
Gender, n (%) |
|
|
0.069 |
|
Male |
8 (10.7) |
16 (21.6) |
|
|
Female |
67 (89.3) |
58 (78.4) |
|
|
Baseline BMI (kg/m2) |
33.7±3.3 |
34.7±53.6 |
0.068 |
|
Comorbidity, n (%) |
|
|
|
|
T2DM |
5 (6.7) |
11 (14.9) |
0.106 |
|
Hypertension |
7 (9.3) |
15 (20.3) |
0.060 |
|
Dyslipidemia |
11 (14.7) |
26 (35.1) |
0.004 |
|
NAFLD |
26 (34.7) |
34 (45.9) |
0.160 |
|
OSAS |
4 (5.3) |
12 (16.2) |
0.064 |
|
Dysmenorrhea |
4/66 (6.1)*
|
7/56 (12.5)*
|
0.216 |
|
Hiatal hernia, n (%)†
|
1 (1.3) |
6 (8.1) |
0.051 |

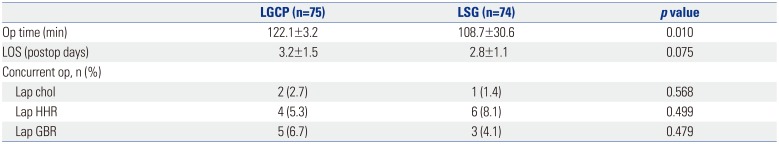
Mean operation time was significantly longer in group A (122.1±3.2 min vs. 108.7±30.6 min,
p=0.010), although mean hospital stays were not significantly different (3.2±1.5 and 2.8±1.1 days,
p=0.075). Laparoscopic cholecystectomy was concurrently performed in 2 (2.7%) patients in group A and 1 (1.4%) patient in group B. Laparoscopic repair of hiatal hernia was concurrently performed in 4 (5.3%) patients in group A, and in 6 (8.1%) patients in group B. Laparoscopic removal of a gastric band was concurrently performed in 5 (6.7%) patients in group A and in 3 (4.1%) patients in group B (
Table 2).
Table 2
Perioperative Data of Patients (n=149)
|
LGCP (n=75) |
LSG (n=74) |
p value |
|
Op time (min) |
122.1±3.2 |
108.7±30.6 |
0.010 |
|
LOS (postop days) |
3.2±1.5 |
2.8±1.1 |
0.075 |
|
Concurrent op, n (%) |
|
|
|
|
Lap chol |
2 (2.7) |
1 (1.4) |
0.568 |
|
Lap HHR |
4 (5.3) |
6 (8.1) |
0.499 |
|
Lap GBR |
5 (6.7) |
3 (4.1) |
0.479 |

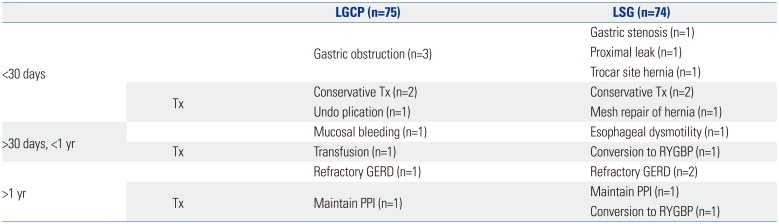
Readmission within 30 days of operation was necessary in 3 patients in both groups. In group A, 3 patients presented with gastric obstruction: two were re-hospitalized after discharge on postoperative days 5 and 7, and both responded well after conservative treatment and a tolerated liquid diet. The third patient who underwent concurrent removal of a gastric band did not improve after conservative treatment and eventually underwent plication reversal at 12 days postoperatively. In group B, 1 patient that presented with gastric obstruction (attributed to a staple mishap during surgery) after discharge was re-hospitalized at 5 days postoperatively and did well after conservative treatment. Another patient presented with severe fever and abdominal pain after discharge on postoperative day 7 and was re-hospitalized. A contained proximal minor leak was diagnosed, and the patient did well after conservative treatment. The third patient presented with frequent bilious vomiting after discharge and was re-hospitalized on postoperative day 5. A hernia was diagnosed at the 15 mm umbilical port site by abdominal CT, and the patient underwent laparoscopic repair with Gore-Tex mesh fixation. One patient in each group was re-hospitalized after 30 days postoperatively. The one patient in group A, did well until 8 months, but then developed melena and syncope. Endoscopy resulted in a positive test for
Helicobacter pylori, but no bleeding focus was found in the plicated stomach. Transfusion of four packed RBCs was started along with Helicobacter eradication. The one patient in group B presented with frequent postprandial chest pain, especially after solids. Esophageal dysmotility was diagnosed by UGI and high resolution manometry, and eventually, the patient underwent conversion to RYGBP at 11 months postoperatively; her symptom subsequently disappeared. After the first postoperative year, one patient in group A and two patients in group B were re-hospitalized for persistent symptomatic gastroesophageal reflux disease (GERD). Of these three patients, the one in group B underwent conversion to RYGBP at 15 months postoperatively (
Table 3).
Table 3
Immediate and Long-Term Postoperative Complications Requiring Readmission (n=149)
|
|
LGCP (n=75) |
LSG (n=74) |
|
<30 days |
|
Gastric obstruction (n=3) |
Gastric stenosis (n=1) |
|
Proximal leak (n=1) |
|
Trocar site hernia (n=1) |
|
Tx |
Conservative Tx (n=2) |
Conservative Tx (n=2) |
|
Undo plication (n=1) |
Mesh repair of hernia (n=1) |
|
>30 days, <1 yr |
|
Mucosal bleeding (n=1) |
Esophageal dysmotility (n=1) |
|
Tx |
Transfusion (n=1) |
Conversion to RYGBP (n=1) |
|
>1 yr |
|
Refractory GERD (n=1) |
Refractory GERD (n=2) |
|
Tx |
Maintain PPI (n=1) |
Maintain PPI (n=1) |
|
Conversion to RYGBP (n=1) |

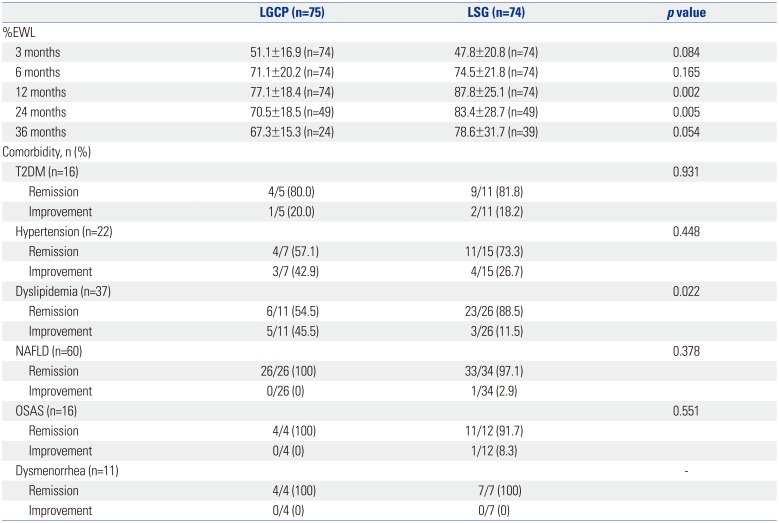
The mean±SD of %EWL in groups A and B at 3, 6, 12, 24, and 36 months were 51.1±16.9 (n=74) and 47.8±20.8 (n=74) (
p=0.084), 71.1±20.2 (n=74) and 74.5±21.8 (n=74) (
p=0.165), 77.1±18.4 (n=74) and 87.8±25.1 (n=74) (
p=0.002), 70.5±18.5 (n=49) and 83.4±28.7 (n=49) (
p=0.005), and 67.3±15.3 (n=24) and 78.6±31.7 (n=39) (
p=0.054), respectively. Mean %EWLs were significant in both groups after 6 months postoperatively (
Table 4,
Fig. 1). Resolution rates of metabolic comorbidities in groups A and B [type 2 diabetes mellitus (T2DM): 4/5 (80%) and 9/11 (81.8%), hypertension: 4/7 (57.1%) and 11/15 (73.3%), and non-alcoholic fatty liver disease: 26/26 (100%) and 33/34 (97.1%)] were not significantly different, except for dyslipidemia [6/11 (54.5%) and 23/26 (88.5%), respectively;
p=0.022]. OSAS resolution rates in the two groups were 4/4 (100%) and 11/12 (91.7%), respectively (
p=0.551). In all 11 patients affected, postoperative dysmenorrhea resolved after significant weight loss [4/4 (100%) vs. 7/7 (100%), respectively] (
Table 4).
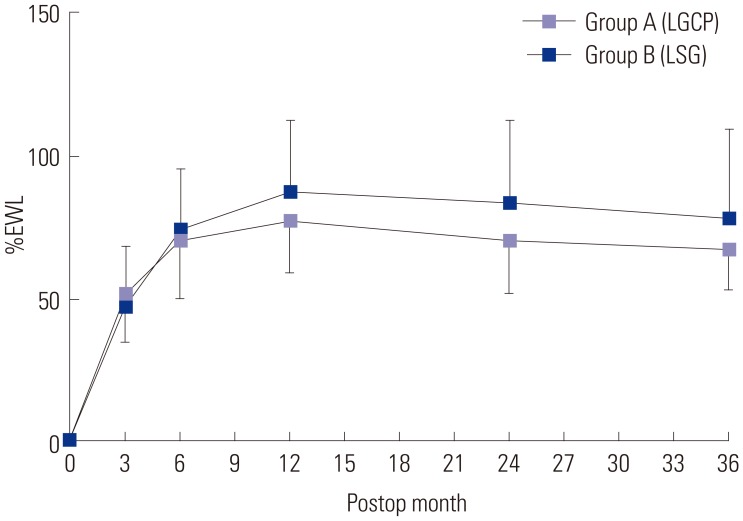 | Fig. 1The mean±SDs percent excess weight losses (%EWL) in groups A and B at 3, 6, 12, 24, and 36 months postoperatively were 51.1±16.9 (n=74) and 47.8±20.8 (n=74) (p=0.084), 71.1±20.2 (n=74) and 74.5±21.8 (n=74) (p=0.165), 77.1±18.4 (n=74) and 87.8±25.1 (n=74) (p=0.002), 70.5±18.5 (n=49) and 83.4±28.7 (n=49) (p=0.005), and 67.3±15.3 (n=24) and 78.6±31.7 (n=39) (p=0.054), respectively. In both groups, the difference in mean %EWL was significant after six months postoperatively. Direction of error bar was below in group A and above in group B. LGCP, laparoscopic greater curvature plication; LSG, laparoscopic sleeve gastrectomy.
|
Table 4
Weight Loss and Changes in Comorbidities after Surgery (n=149)
|
LGCP (n=75) |
LSG (n=74) |
p value |
|
%EWL |
|
|
|
|
3 months |
51.1±16.9 (n=74) |
47.8±20.8 (n=74) |
0.084 |
|
6 months |
71.1±20.2 (n=74) |
74.5±21.8 (n=74) |
0.165 |
|
12 months |
77.1±18.4 (n=74) |
87.8±25.1 (n=74) |
0.002 |
|
24 months |
70.5±18.5 (n=49) |
83.4±28.7 (n=49) |
0.005 |
|
36 months |
67.3±15.3 (n=24) |
78.6±31.7 (n=39) |
0.054 |
|
Comorbidity, n (%) |
|
|
|
|
T2DM (n=16) |
|
|
0.931 |
|
Remission |
4/5 (80.0) |
9/11 (81.8) |
|
|
Improvement |
1/5 (20.0) |
2/11 (18.2) |
|
|
Hypertension (n=22) |
|
|
0.448 |
|
Remission |
4/7 (57.1) |
11/15 (73.3) |
|
|
Improvement |
3/7 (42.9) |
4/15 (26.7) |
|
|
Dyslipidemia (n=37) |
|
|
0.022 |
|
Remission |
6/11 (54.5) |
23/26 (88.5) |
|
|
Improvement |
5/11 (45.5) |
3/26 (11.5) |
|
|
NAFLD (n=60) |
|
|
0.378 |
|
Remission |
26/26 (100) |
33/34 (97.1) |
|
|
Improvement |
0/26 (0) |
1/34 (2.9) |
|
|
OSAS (n=16) |
|
|
0.551 |
|
Remission |
4/4 (100) |
11/12 (91.7) |
|
|
Improvement |
0/4 (0) |
1/12 (8.3) |
|
|
Dysmenorrhea (n=11) |
|
|
- |
|
Remission |
4/4 (100) |
7/7 (100) |
|
|
Improvement |
0/4 (0) |
0/7 (0) |
|

Go to :

DISCUSSION
The main findings of the present study concern weight loss and the complications of LGCP and LSG performed on obese patients with BMIs ranging from 30 to 35 kg/m
2. Although mean %EWL in LGCP was inferior to that of LSG, especially after six months postoperatively [%EWL at 12, 24, and 36 months in groups A and B were 77.1 and 87.8% (
p=0.002), 70.5 and 83.4% (
p=0.005), and 67.3 and 78.6% (
p=0.054), respectively], and these values were acceptable. Also, LGCP achieved an excellent metabolic comorbidity resolution rate. T2DM of the five patients in LGCP group in our study were a relatively mild form of T2DM [diagnosed in preoperative evaluation (HbA1C) or controlled by oral hypoglycemic <2 years]. Therefore, the resolution rate of T2DM in LGCP in our study was relatively high, which is contrary to the previous studies showing resolution of comorbidities, especially in T2DM is less favorable in LGCP. The observed %EWL inferiority was probably due to the anatomic changes typical of LGCP, as LGCP preserves more of the stomach wall after infolding the greater curvature, and this is prone to relaxation with time and results in dilatation of the plicated stomach.
56789 Few studies have investigated the effectiveness of LGCP in patients with BMIs in the 30 to 35 kg/m
2 range. Skrekas, et al.,
13 in a series of 135 patients, found %EWL was significantly higher for LGCP patients with a BMI of <45 kg/m
2 than for patients with a BMI of >45 kg/m
2 (69.86% vs. 55.49%). Mui, et al.
8 presented a series of 20 LGCP patients with BMIs of <35 and reported mean %EWL values of 76.5, 76.5, and 65.0% at 3, 6, and 12 months postoperatively. Kim, et al.
10 presented their findings of 64 patients with a mean BMI of 31.4 kg/m
2 and reported %EWL values of 34.7, 50.8, 61.1, 82.1, and 82.9% at 1, 3, 6, 12, and 18 months, respectively. Shen, et al.
9 conducted a study on 22 patients with a mean of BMI 37.0 kg/m
2, and at 1, 3, 6, and 12 months postoperatively, LGCP was found to produce mean %EWL values of 22.9, 38.6, 51.5, and 61.1%, respectively. Based on the above results and our findings, we are of the opinion LGCP should be performed in less obese patients who require less weight loss. In fact, most procedures have better weight loss in low BMI patients, including LGCP, LSG, and even bypass. Therefore, it would be more logical to choose LGCP over LSG in this group of patient unless the patient has severe comorbidities. Those who do not want gastric stapling/resection, or to whom sleeve gastrectomy is not affordable, also can benefit from LGCP.
Regarding postoperative complications, LGCP and LSG showed the same incidences of immediate and long-term complications in the present study. Three cases of gastric obstruction (3/75, 4%) occurred among LGCP patients, and conservative management, including intravenous hydration and
nil per os, were sufficient to correct these problems. However, one LGCP patient (1/75, 1.3%) underwent plication reversal at 12 days postoperatively. Gastric obstruction requiring revision has been constantly reported in large-scale LGCP studies, with rates ranging from 0.4 to 7.7%.
1415 The definition of gastric obstruction has not been defined, and no guidelines have been issued regarding the duration of conservative management, because gastric obstruction after LGCP usually subsides in parallel with edema of the apposed gastric wall. From a technical point of view, narrowing (distortion) of His angle or incisura angularis should be avoided to minimize gastric obstruction. In group B, all three immediate postoperative complications, that is, gastric stenosis after a stapling mishap during surgery, proximal leak, and an umbilical 15-mm trocar site hernia after specimen removal, were attributed to gastric stapling and were, thus, specific complications of LSG. No case of gastric prolapse during the immediate postoperative period was encountered. However, in each group, a rare complication occurred after postoperative 30 days. One involved delayed gastric bleeding at 8 months after LGCP, and in this case, no bleeding focus was identified endoscopically or angiographically. We speculate bleeding was caused by a small, Dieulafoy-like, mucosal lesion around non-absorbable suture material that had entered the gastric lumen during plication. Conservative management should be the priority for this complication. The second rare complication of esophageal dysmotility was diagnosed in a patient in the LSG group. This patient presented severe odynophagia after solid intake, which was corrected after conversion to gastric bypass. We postulate this phenomenon was caused by malfunction of the esophageal motor function due to a small, narrowed, tubular stomach after sleeve resection. This case indicated esophageal dysmotility as another functional complication specific to gastric stapling in LSG.
The last issue concerns postoperative GERD, which was a common symptom in both groups for several months following surgery. High intraluminal pressure caused by a narrow, tube like stomach and an intact pylorus sphincter may play major roles in the development of GERD after either procedure. However, the underlying mechanism of GERD appears to differ after LGCP or LSG. Kim and Kim
11 suggested that GER after LGCP is due to high intraluminal pressure and resulting ‘transient LES insufficiency’ rather than a damaged anti-reflux mechanism resulting from gastric resection during LSG. In our clinical experience, many patients complain of reflux symptom for several months after LGCP, but few complain of persistent symptomatic GERD. Education about eating skills and the use of proton pump inhibitors (PPIs) and antiemetics usually resolve these problems, but in one patient in group A and in two patients in group B, postoperative PPI could not be discontinued during the first postoperative year. Furthermore, one patient in the LSG group underwent conversion to RYGBP for symptomatic relief. Postop volvulus and adhesion may also be responsible for GERD after LSG. Gastrosplenic, gastrophrenic, gastrocolic, and gastrohepatic ligaments hold the stomach in its anatomical position, and thus, the stomach may be prone to volvulus whenever gastric fixation is lax or the stomach is incorrectly positioned after surgical manipulation. Twisting of the gastric remnant is a condition similar to organo-axial gastric volvulus. Due to the intentional creation of a narrow tube-like stomach, LSG may result in a twisted or spiral sleeve caused by progressive rotation of the staple line in an anterior to posterior plane, and this can lead to functional narrowing, despite a fairly normal luminal diameter.
16
Several limitations of the present study warrant mentioning. First, the study had a retrospective design and the cohort was relatively small, especially after 3 years postoperatively. Nevertheless, this is the first mid-term comparative study to be conducted on LSG and LGCP with a follow-up of up to 36 months, and despite its size, the cohort was larger than that of previous studies on similar topics. Second, the BMIs of patients enrolled in the present study were relatively low (<40 kg/m
2), because, in Korea, the number of superobese and morbidly obese patients is relatively small, which appears to be why our results relatively favor LGCP over LSG and why they differ from those of other comparative studies on patients with BMIs exceeding 40 kg/m
2. However, our observations are in line with many observational studies that concluded LGCP is maximally effective in obese patients with a lower BMI. Accordingly, our results should not be applied to the superobese or morbidly obese. Third, asymptomatic radiologic problems, such as, neofundus formation, which we regard as normal adaptation of the upper part of stomach after LGCP, were not well described in the present study. Verdi, et al.
5 concluded gastric prolapse was the major cause of revision after LGCP with one revision (2.2%) for acute prolapse and 15 revisions (33.3%) for later gastric prolapse among 45 patients. We view fixation of the entire fundus using multiple plication sutures in the inner row of sutures as critical. In addition, we find non-absorbable knotless unidirectional barbed suture material (V-Loc) is best suited for the outer row of sutures, because they are not easily loosened during continuous suture reinforcement. With the exception of one patient (described in the Results), we had no long-term complaint of chronic abdominal pain or prolonged GER suggestive of ‘pathologic’ neofundus.
In conclusion, the present study shows that mean weight loss after LGCP, in patients with BMIs ranging from 30 to 35 kg/m2, is inferior to that after LSG, especially after the first six postoperative months. Nonetheless, weight losses were still acceptable, the resolution rate of metabolic comorbidities was excellent, and acute and chronic complication rates were satisfactory and comparable to LSG during the first three postoperative years.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download