Abstract
Purpose
During emergence from anesthesia for a craniotomy, maintenance of hemodynamic stability and prompt evaluation of neurological status is mandatory. The aim of this prospective, randomized, double-blind study was to compare the effects of dexmedetomidine and remifentanil on airway reflex and hemodynamic change in patients undergoing craniotomy.
Materials and Methods
Seventy-four patients undergoing clipping of unruptured cerebral aneurysm were recruited. In the dexmedetomidine group, patients were administered dexmedetomidine (0.5 µg/kg) for 5 minutes, while the patients of the remifentanil group were administered remifentanil with an effect site concentration of 1.5 ng/mL until endotracheal extubation. The incidence and severity of cough and hemodynamic variables were measured during the recovery period. Hemodynamic variables, respiration rate, and sedation scale were measured after extubation and in the post-anesthetic care unit (PACU).
Results
The incidence of grade 2 and 3 cough at the point of extubation was 62.5% in the dexmedetomidine group and 53.1% in the remifentanil group (p=0.39). Mean arterial pressure (p=0.01) at admission to the PACU and heart rate (p=0.04 and 0.01, respectively) at admission and at 10 minutes in the PACU were significantly lower in the dexmedetomidine group. Respiration rate was significantly lower in the remifentanil group at 2 minutes (p<0.01) and 5 minutes (p<0.01) after extubation.
During recovery from an intracranial procedure, smooth emergence from general anesthesia with hemodynamic stability is a major concern for the anesthesiologist. Airway reflex and systemic hypertension during recovery may lead to cerebral edema or intracranial hemorrhage.12 Furthermore, rapid recovery from general anesthesia is imperative in aiding early detection of potential intracranial complications and neurologic deficits.
A variety of strategies have been proposed for rapid and smooth emergence from general anesthesia.345678 Remifentanil is an ultra-short-acting opioid and is conventionally used as an adjuvant agent in general anesthesia. Previous studies have shown that remifentanil prevents airway response and detrimental hemodynamic changes without a significant increase in recovery time.6910 However, in neurosurgical patients, possible respiratory depression and related hypercapnia may be of concern when maintaining remifentanil target controlled infusion (TCI) during the recovery period from general anesthesia.
Dexmedetomidine is a potent, alpha 2-selective adrenoceptor agonist that causes sympatholysis, sedation, and analgesia without inhibiting respiration.1112 Several studies have reported that dexmedetomidine reduced hypertension and tachycardia in patients undergoing neurosurgery.1314 To the best of our knowledge, there have only been limited studies that have compared recovery profiles between a single dose of dexmedetomidine and remifentanil TCI in neurosurgical patients.1516
Therefore, we hypothesized that a single dose of dexmedetomidine can be more effective than remifentanil in attenuating airway reflexes and hemodynamic changes during the recovery period. The purpose of this prospective, randomized, casecontrolled study was to compare the effects of dexmedetomidine and remifentanil on airway reflexes and hemodynamic changes during the recovery period in patients undergoing craniotomy.
After obtaining approval from the Severance Hospital Institutional Review Board (ref: 1-2011-0019), the study was registered at ClinicalTrials.gov (ref: NCT01365923). Written informed consent was obtained from all patients. Seventy-four patients, who were 20–70 years old, American Society of Anesthesiologists class I–II, and scheduled for clipping of an unruptured cerebral aneurysm (Modified Hunt and Hess Clinical grade 0), were prospectively enrolled in this study. Patients with respiratory disease, advanced heart block, or uncontrolled hypertension were excluded from the study. Patients were randomly allocated to one of two groups, the dexmedetomidine group or the remifentanil group, using an Excel®-generated randomization table.
No patients were premedicated on arrival to the operating room. Patients were monitored via electrocardiography, pulse oximetry, non-invasive blood pressure, and capnography. General anesthesia was induced with propofol 1.5 mg/kg and remifentanil at an effect site concentration (Ce) of 4 ng/mL. The remifentanil infusion rate was controlled using a commercial TCI system (Orchestra Base Primea, Fresenius Vial, Brezins, France) incorporating Minto's pharmacokinetic model.17 After rocuronium 0.6 mg/kg was given intravenously, patients were intubated using an endotracheal tube with an internal diameter of 7.5 mm (for males) or 6.5 mm (for females). A cuff was inflated to maintain pressure between 20–25 cm H2O measured using a manometer (Hi-Lo™ Hand Pressure Gauge, VBM Medizintechnik GmbH, Sulz am Neckar, Germany). A 20-G catheter was inserted in the radial artery to monitor arterial blood pressure and arterial blood gas. A forced-air warming system (Bair-Hugger™, Augustine-Medical, Eden Prairie, MN, USA) was applied throughout surgery to maintain the body temperature at 36.0–37.0℃.
Anesthesia was maintained with 0.6–1 minimum alveolar concentration (MAC) end-tidal concentration of sevoflurane in 50% oxygen/air mixture by using the Bispectral Index (BIS VISTA Monitoring System Inc., Norwood, MA, USA) to maintain a target BIS of 45–55. The remifentanil Ce was adjusted to sustain a mean arterial pressure (MAP) and heart rate within 20% of preoperative resting values. All patients were positioned to optimize cerebral venous drainage, and mechanical ventilation was adjusted to maintain PaCO2 35–40 mm Hg, confirmed during the operation via arterial blood gas analysis.
Ten minutes before the end of each surgery, sevoflurane was titrated to 0.6 MAC, and remifentanil was titrated to a Ce of 1.5 ng/mL in both groups. In the dexmedetomidine group, 0.5 µg/kg dexmedetomidine was infused for 5 minutes. After the termination of dexmedetomidine administration, a nurse, who concealed the group from the investigator, switched a syringe of remifentanil to normal saline. Patients in the two groups received continuous infusion of remifentanil or normal saline at a Ce of 1.5 ng/mL until extubation. In both groups, after all surgical procedures were finished, sevoflurane and air-flow were discontinued, and oxygen flow was increased to 10 L/min. Another anesthesiologist, blinded to the randomization, performed the remaining anesthetic recovery processes and collected all data. Ramosetron 0.3 mg was administered for the prevention of postoperative nausea and vomiting (PONV). Respiration was assisted by manual ventilation after confirming neuromuscular function via the train-of-four response. If patients opened their eyes in response to a verbal request and their respiration was adequate, the trachea was extubated after cuff deflation. In both groups, the blinded syringe of remifentanil or normal saline was discontinued immediately after extubation. Patients in both groups were monitored while receiving 100% oxygen for 5 minutes. Then the patients were transferred to the post-anesthetic care unit (PACU). When patients requested to receive rescue analgesics and the postoperative pain score assessed using a visual analogue scale was above 5 points, fentanyl 1 µg/kg was administered.
The primary outcome was a coughing response, which was evaluated during the recovery period from the time of awareness to 5 minutes after extubation. Coughing severity was classified using the 3-point scale described by Minogue, et al.:5 1=mild (single) cough, 2=moderate (≤5 s) cough, and 3=severe (>5 s) cough. Grades 2 and 3 were considered clinically deleterious. MAP and heart rate values were recorded 5 minutes before the end of surgery (T1), at the end of surgery (T2), at the point of awareness (T3), at the point of extubation (T4), 2 minutes after extubation (T5), 5 minutes after extubation (T6), at admission to the PACU (T7), and 10 minutes after admission to the PACU (T8). The respiration rate and sedation were recorded at T5, T6, T7, and T8. Sedation was classified using a 4-point scale: 0=sleepy and not arousable, 1=sleepy yet arousable, 2=drowsy, and 3=alert.18 Time to awareness (from the end of the surgery to eyes opening) and time to extubation (from the end of the surgery to extubation) were also recorded and compared. Postoperative pain score, consumption of analgesics, PONV, and any adverse events at the PACU were recorded.
We calculated the sample size based on the previous finding that 1.5 ng/mL of remifentanil suppressed coughing in 69% of patients under sevoflurane-remifentanil anesthesia7 and that a difference in the incidence of at least 30% would be clinically relevant. Thus, 31 patients per group were required based on α=0.05. Assuming a loss of follow-up of 20% of the patients, a total sample size of 74 was needed to achieve 80% power. Statistical analyses were performed using the SAS software package, version 9.2 (SAS Institute Inc., Cary, NC, USA). Coughing incidence and severity were analyzed using the Mantel-Haenszel chi-squared test. After testing for normally distributed data using the Kolmogorov-Smirnov test and Shapiro-Wilk test, continuous and categorical variables were analyzed using a two-sample t-test and a chi-squared test, respectively. The variables that did not show normal distribution were analyzed using a Mann-Whitney U test. Student's t-test at each time point and a general linear mixed model were used when comparing changes in MAP, heart rate, and respiration rate between the two groups. The sedation scale was analyzed using a generalized estimating equation. A p value of <0.05 was considered statistically significant.
Of the 74 patients enrolled in this study, eight were excluded due to thiopental sodium administration for brain protection during temporary clipping, and two patients were withdrawn due to surgical bleeding. Therefore, 64 patients successfully completed the study, and their characteristics can be found in Table 1.
There were no significant differences between groups in terms of the incidence (p=0.14) and severity (p=0.39) of coughing (Fig. 1). At the point of extubation, 18 patients (56.2%) in the dexmedetomidine group and 12 patients (37.5%) in the remifentanil group presented grade 2 coughs while two patients (6.3%) in the dexmedetomidine group and five patients (15.6%) in the remifentanil group presented grade 3 coughs.
Fig. 2 shows the MAPs and heart rates of both groups over time. MAP (p=0.01) and heart rate (p=0.04 and 0.01, respectively) at admission to PACU and after 10 minutes in the PACU were significantly higher in the remifentanil group than in the dexmedetomidine group. The respiration rate was significantly lower in the remifentanil group than in the dexmedetomidine group at 2 minutes (p<0.01) and 5 minutes (p<0.01) after extubation (Fig. 3). The recovery profiles of both groups are shown in Table 2. There was no significant difference between the two groups in sedation score. Time to awareness in the dexmedetomidine group was longer than in the remifentanil group yet not statistically significant (p=0.05). Postoperative pain score (p=0.57) and consumption of analgesics (p=0.59) did not differ between the two groups. No adverse events occurred in either group.
In the present study, a single dose of dexmedetomidine was as effective as remifentanil TCI in attenuating airway reflex, hypertension, and tachycardia during recovery from general anesthesia in patients undergoing clipping of an unruptured cerebral aneurysm. In addition, such a dose did not appear to induce respiratory depression. The respiration rate was significantly higher in the dexmedetomidine group than in the remifentanil group at 2 and 5 minutes after extubation.
Rapid recovery of consciousness while maintaining hemodynamic stability is considered more important for neurosurgery than any other surgical area. Therefore, various agents have been investigated to allow for smooth emergence after craniotomy. Previous studies demonstrated that remifentanil can be useful based on its sensitive half-time, allowing for a more predictable emergence and recovery than other opiate agents.192021 Therefore, we chose a Ce of 1.5 ng/mL, which is close to the EC50 of remifentanil in suppressing emergence cough,6 and compared it to the antitussive effect of dexmedetomidine. Jun, et al.7 reported that 1.5 ng/mL of remifentanil effectively reduced emergence cough after sevoflurane-remifentanil anesthesia. The dexmedetomidine dose was based on a study that concluded that a single, 0.5 µg/kg of dose of dexmedetomidine attenuates noxious stimuli when compared to a placebo group.1122
Most sedatives, hypnotics, and analgesics suppress the stimulatory effect of hypoxemia or hypercapnia on ventilation. Therefore, excessive administration of these drugs can produce respiratory depression, which significantly exacerbates hypoxia or CO2 retention. While hypoxia and hypercapnia can be deleterious in all surgical fields, it must be particularly avoided in patients with intracranial pathology, as events of hypoxia and hypercapnia may eventually lead to the aggravation of poor neurologic outcomes. Rapid recovery of consciousness is critical for the postoperative period after neurosurgery. Delayed emergence could confuse the neurosurgeon and anesthesiologist. The recovery period after neurosurgery requires a special strategy to ensure smooth emergence without respiratory depression and delayed sedation.
There are many articles regarding sedation with dexmedeto midine that describe the distinctive qualities of sedation, which is similar to normal sleep. Particularly in intensive care units, dexmedetomidine induces sedation while allowing patients to interact and answer questions when stimulated yet return to tranquility and calmness when left alone.1323 This unique characteristic of sedation can allow for a smooth emergence from general anesthesia by blunting airway reflexes, agitation, and hemodynamic changes.
Hsu, et al.24 demonstrated that respiration changes occur during remifentanil infusion, as the tidal volume does not increase enough to overcome the reduction of the respiration rate; as such, the decrease of minute ventilation can produce hypercapnia. On the other hand, the respiration pattern during dexmedetomidine infusion is virtually unchanged. This preserved respiration is a unique advantage of the α-2 agonist that distinguishes it from other sedatives, hypnotics, and analgesics.2425 The present study showed that patients who were treated with dexmedetomidine maintained an appropriate respiration rate from extubation to 5 minutes thereafter, which supports the respiration property of dexmedetomidine.24 Even if the effects of remifentanil disappear rapidly, transiently depressed respiration in the immediate postoperative period can occur and worsen the neurologic outcome.26 In patients with a higher risk of postoperative respiratory depression and in those who have undergone surgery and require extreme caution regarding respiratory depression, administering dexmedetomidine is preferable to opioid analgesics.122728
In the recovery period after general anesthesia, dexmedetomidine can attenuate coughing reflex and prevent emergence agitation when compared with opiate agents.2930 A recent study reported that an additional single dose (0.5 µg/kg) of dexmedetomidine can attenuate emergence cough when compared with an infusion of low-dose remifentanil alone without delaying recovery from general anesthesia. A single dose of dexmedetomidine can help smooth emergence without respiratory depression and delayed sedation.22
As mentioned above, intravenous dexmedetomidine is effective in inhibiting hypertension and tachycardia during the perioperative period for neurosurgical patients. On the other hand, there are previous studies that have reported an increase of systemic arterial pressure in response to rapid intravenous administration of dexmedetomidine.3132 This study, which involved the administration of 0.5 µg/kg dexmedetomidine for 5 minutes, did not result in an increase of blood pressure or the use of antihypertensive medications when compared to remifentanil. When the patient was admitted to the PACU, MAP and heart rate were significantly lower in patients who received dexmedetomidine. Moreover, 10 minutes after admission to the PACU, heart rate was significantly lower in patients who received dexmedetomidine. This difference was statistically significant yet within the clinically acceptable range.
The incidence of coughing in the present study was slightly higher than those of previous studies.78 We speculate that the difference in coughing response might have been due to the type of surgeries, features of patients, and recovery maneuvers during extubation. Furthermore, it might have also been due to applying coughing criteria strictly, as we considered even small movements of the head as coughing. Additionally, unnecessary deeper sedation with the purpose of suppressing coughing could be dangerous for neurosurgical patients. Excessive sedation can disrupt prompt detection of neurologic change and induce hypoventilation and hypercapnia, and the neurologic outcome can be worsened. Although the mechanism of the antitussive effect of dexmedetomidine is not clear in the previous studies, which evaluated the effects of dexmedetomidine in the recovery period after general anesthesia, the sedation property without respiratory depression may play a major role in the attenuation of the response related to extubation.
There were several limitations to this study. First, as the exact data on hypoventilation or hypercapnia were not obtained during the recovery periods, it was not clear whether or not significant respiratory depression was present in the remifentanil group. We previously demonstrated that most patients receiving a Ce of over 2 ng/mL of remifentanil showed hypercapnia (end tidal CO2 concentration >40 mm Hg) during anesthetic emergence.33 Although respiratory rate is not equivalent to minute ventilation and it is not evident whether opioids induce clinically significant respiratory depression in neurosurgical patients, systemically administered opioids profoundly diminish the respiratory rate more so than the reduction of tidal volume, which may induce hypercapnia.34 Thus, remifentanil administration can induce hypercapnia, and it may present a potential risk in patients undergoing craniotomy. Second, remifentanil and dexmedetomidine are different agents with distinct mechanisms, and there is no existing research regarding the optimal dexmedetomidine and remifentanil doses to suppress the airway reflexes after a craniotomy. Therefore, determining reliable parameters for equipotent concentrations at each time is difficult. Residual effects of drugs that affect airway reflexes determine the extent of the coughing response before and after extubation. However, in this study, there were no differences between groups in terms of end-tidal sevoflurane concentration. In patients treated with a single bolus of dexmedetomidine, the Ce of remifentanil was so low that it could not play any clinical role.
In conclusion, a single bolus of dexmedetomidine and remifentanil TCI have equal effectiveness in attenuating airway reflexes and hemodynamic changes in patients undergoing cerebral aneurysm clipping, although better preservation of respiration was observed in the dexmedetomidine group.
Figures and Tables
Fig. 1
Cough profile at the point of awareness (A) and extubation (B) for the dexmedetomidine group (□) and remifentanil group (▪). Grade of cough: 0=no cough, 1=mild (single) cough, 2=moderate (≤5 s) cough, 3=severe (>5 s) cough.

Fig. 2
Changes in mean arterial pressure and heart rate during recovery for the dexmedetomidine group (•) and remifentanil group (□). *p<0.05 between groups. T1, 5 minutes before end of surgery; T2, at termination of sevoflurane; T3, at awareness; T4, at extubation; T5, 2 minutes after extubation; T6, 5 minutes after extubation; T7, at admission to PACU; T8, 10 minutes in PACU. PACU, post-anesthetic care unit.

Fig. 3
Changes in respiration rate after extubation for the dexmedetomidine group (•) and remifentanil group (□). *p<0.05 between groups. T5, 2 minutes after extubation; T6, 5 minutes after extubation; T7, at admission to PACU; T8, 10 minutes in PACU. PACU, post-anesthetic care unit.

Table 1
Patient Characteristics and Clinical Data
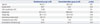
Table 2
Recovery Profiles

ACKNOWLEDGEMENTS
The authors thank Ha Yan Kim, Biostatistician, Department of Research Affairs, Yonsei University College of Medicine, for assisting in the statistical analysis.
This research was supported by a special research grant funded by the Korean Society of Neuroscience in Anesthesiology and Critical Care (KSNACC-2012).
Notes
References
1. Leech P, Barker J, Fitch W. Proceedings: changes in intracranial pressure and systemic arterial pressure during the termination of anaesthesia. Br J Anaesth. 1974; 46:315–316.

2. Irwin RS. Complications of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129:1 Suppl. 54S–58S.
3. Gefke K, Andersen LW, Friesel E. Lidocaine given intravenously as a suppressant of cough and laryngospasm in connection with extubation after tonsillectomy. Acta Anaesthesiol Scand. 1983; 27:111–112.

4. Mendel P, Fredman B, White PF. Alfentanil suppresses coughing and agitation during emergence from isoflurane anesthesia. J Clin Anesth. 1995; 7:114–118.

5. Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004; 99:1253–1257.

6. Lee B, Lee JR, Na S. Targeting smooth emergence: the effect site concentration of remifentanil for preventing cough during emergence during propofol-remifentanil anaesthesia for thyroid surgery. Br J Anaesth. 2009; 102:775–778.

7. Jun NH, Lee JW, Song JW, Koh JC, Park WS, Shim YH. Optimal effect-site concentration of remifentanil for preventing cough during emergence from sevoflurane-remifentanil anaesthesia. Anaesthesia. 2010; 65:930–935.

8. Lee JH, Koo BN, Jeong JJ, Kim HS, Lee JR. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth. 2011; 106:410–415.

9. Nho JS, Lee SY, Kang JM, Kim MC, Choi YK, Shin OY, et al. Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Br J Anaesth. 2009; 103:817–821.

10. Chen J, Li W, Wang D, Hu X. The effect of remifentanil on cough suppression after endoscopic sinus surgery: a randomized study. Acta Anaesthesiol Scand. 2010; 54:1197–1203.

11. Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005; 15:762–766.

12. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008; 106:1741–1748.

13. Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: a double-blind, randomized and placebocontrolled study. Br J Anaesth. 2006; 97:658–665.

14. Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008; 107:1340–1347.

15. Turgut N, Turkmen A, Ali A, Altan A. Remifentanil-propofol vs dexmedetomidine-propofol--anesthesia for supratentorial craniotomy. Middle East J Anaesthesiol. 2009; 20:63–70.
16. Gunduz M, Gunes Y, Ozbek H, Yilmaz D, Isik G. Comparison of dexmedetomidine or remifentanil infusion combined with sevoflurane anesthesia in craniotomy: hemodynamic variables and recovery. Neurosurg Q. 2009; 19:116–119.

17. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86:10–23.

18. Alon E, Baitella L, Hossli G. Double-blind study of the reversal of midazolam-supplemented general anaesthesia with Ro 15-1788. Br J Anaesth. 1987; 59:455–458.

19. Kapila A, Glass PS, Jacobs JR, Muir KT, Hermann DJ, Shiraishi M, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995; 83:968–975.

20. Guy J, Hindman BJ, Baker KZ, Borel CO, Maktabi M, Ostapkovich N, et al. Comparison of remifentanil and fentanyl in patients undergoing craniotomy for supratentorial space-occupying lesions. Anesthesiology. 1997; 86:514–524.

21. Sneyd JR, Whaley A, Dimpel HL, Andrews CJ. An open, randomized comparison of alfentanil, remifentanil and alfentanil followed by remifentanil in anaesthesia for craniotomy. Br J Anaesth. 1998; 81:361–364.

22. Lee JS, Choi SH, Kang YR, Kim Y, Shim YH. Efficacy of a single dose of dexmedetomidine for cough suppression during anesthetic emergence: a randomized controlled trial. Can J Anaesth. 2015; 62:392–398.

23. Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999; 54:1136–1142.

24. Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004; 101:1066–1076.
25. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000; 93:382–394.

26. Cold GE, Felding M. Even small doses of morphine might provoke "luxury perfusion" in the postoperative period after craniotomy. Neurosurgery. 1993; 32:327.

27. Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth. 2006; 18:24–28.

28. Zhuang PJ, Wang X, Zhang XF, Zhou ZJ, Wang Q. Postoperative respiratory and analgesic effects of dexmedetomidine or morphine for adenotonsillectomy in children with obstructive sleep apnoea. Anaesthesia. 2011; 66:989–993.

29. Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013; 111:222–228.

30. Kavalci G, Ethemoglu FB, Durukan P, Batuman A, Emre C. Comparison of the effects of dexmedetomidine and remiphentanyl on emergence agitation after sevoflurane anesthesia in adults undergoing septoplasty operation: a randomized double-blind trial. Eur Rev Med Pharmacol Sci. 2013; 17:3019–3023.
31. Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans II Hemodynamic changes. Anesthesiology. 1992; 77:1134–1142.
32. Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993; 78:813–820.

33. Choi SH, Min KT, Lee JR, Choi KW, Han KH, Kim EH, et al. Determination of EC95 of remifentanil for smooth emergence from propofol anesthesia in patients undergoing transsphenoidal surgery. J Neurosurg Anesthesiol. 2015; 27:160–166.

34. Catley DM, Thornton C, Jordan C, Lehane JR, Royston D, Jones JG. Pronounced, episodic oxygen desaturation in the postoperative period: its association with ventilatory pattern and analgesic regimen. Anesthesiology. 1985; 63:20–28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download