Abstract
Purpose
To evaluate the technical feasibility and safety of vascular plug assisted retrograde transvenous obliteration (PARTO) for bleeding gastric varix performed in the emergent clinical setting and describe the mid-term clinical results.
Materials and Methods
From April 2012 to January 2015, emergent PARTO was tried in total 9 patients presented with active gastric varix bleeding. After initial insufficient or failure of endoscopic approach, they underwent PARTO in the emergent clinical setting. Gelatin sponge embolization of both gastrorenal (GR) shunt and gastric varix was performed after retrograde transvenous placement of a vascular plug in GR shunt. Coil assisted RTO (CARTO) was performed in one patient who had challenging GR shunt anatomy for vascular plug placement. Additional embolic materials, such as microcoils and NBCA glue-lipiodol mixture, were required in three patients to enhance complete occlusion of GR shunt or obliteration of competitive collateral vessels. Clinical success was defined as no variceal rebleeding and disappearance of gastric varix.
Results
All technical and clinical success–i.e., complete GR shunt occlusion and offending gastric varix embolization with immediate bleeding control–was achieved in all 9 patients. There was no procedure-related complication. All cases showed successful clinical outcome during mean follow up of 17 months (12–32 months), evidenced by imaging studies, endoscopy and clinical data. In 4 patients, mild worsening of esophageal varices or transient ascites was noted as portal hypertensive related change.
Gastro-esophageal varix occurring in liver cirrhosis accompanied by portal hypertension are one of the leading causes of death. Especially in the gastric fundal varix (GV) with active bleeding, the pharmacologic or endoscopic treatments are often difficult.1234 When the endoscopic treatment is limited, the transjugular intrahepatic portosystemic shunt (TIPS) or balloon-occluded retrograde transvenous obliteration (BRTO) may be a treatment indication in terms of vascular interventional procedure.456
Even though BRTO has been shown to have reliable clinical results in GV treatment through a number of studies to date, relatively long procedure time (few hours to overnight) was one of the inherent limitations of indwelling balloon catheter and sclerosing agent combination.78910111213 To overcome the procedure time logistics, recently proposed new concept of BRTO–plug assisted retrograde transvenous obliteration (PARTO) and coil assisted RTO (CARTO)–appears to be distinctive, and it showed acceptable clinical results.14151617 However, a small number of study were so far carried out, and there is no study, focusing these modified techniques onto active GV bleeding patients in the emergent clinical setting.
Thus, this study was carried out to evaluate the feasibility and safety of PARTO that was performed on GV bleeding patients under an emergent clinical setting and analyze the mid-term clinical results.
This retrospective study was conducted after getting the approval of the Institutional Review Board and informed consent was obtained from each patient. The modified BRTO (PARTO n=8/CARTO n=1) procedures were performed on total 9 patients (5 men and 4 women; age range 51–72 years; mean age, 59 years) from April 2012 to January 2015 at our hospital. The demographics of the patients are outlined in Table 1. Total 8 patients came to our emergency center with gastrointestinal (GI) bleeding signs such as massive hematemesis, hematochezia, and melena. Their conditions were evaluated to be difficult or insufficient to apply endoscopic therapy–mostly due to poor endoscopic visual field with massive hemorrhage, therefore, vascular interventions procedure has been requested. Out of these 8 patients, 4 patients had a previous history of having received endoscopic treatments due to GV bleeding. One patient undertook the procedure due to the GV bleeding occurred during hospitalization for treatment of liver cirrhosis and grade I hepatic encephalopathy (West Haven Criteria). Modified BRTO procedures were performed within 2 hours after failing an emergent endoscopic treatment trial in all patients.
An anatomical evaluation and classification of GV and gastrorenal (GR) shunt were performed in all patients through endoscopy and CT findings before the procedure and a classification of GR shunt was analyzed using retrograde venography that had been performed during the procedures.57181920 The evaluation was made in line with the classification pursuant to the criteria of Hirota, et al.7–grade 1 (n=5), grade 2 (n=3), grade 3 (n=1); isolated gastric varix type 1 (IGV1; n=5), gastroesophageal varix type 2 (GOV2; n=4) classification was made in accordance with endoscopy findings and CT findings. The approximate shape and diameter of GR shunt were evaluated using multiplanar reformation CT images.
The procedure was performed after inducing conscious sedation using an intravenous pethidine hydrochloride (Demerol; Keukdong Pharmaceuticals, Seoul, Korea) and local anesthesia using 2% lidocaine (Keukdong Pharmaceuticals, Seoul, Korea). Access to GR shunt was made through a transfemoral approach in all patients. In one patients, transjugular approach was initiated at first, but switched to a transfemoral approach due to the anatomical difficulty of selection of GR shunt and advancing the guiding sheath. After left renal vein and GR shunt were selected using 5 Fr Cobra or Simons shaped angiocatheter and 180 cm 0.035 inch guide wire (Terumo, Tokyo, Japan), over the wire-guiding sheath exchanging (Flexor Check-Flo or shuttle sheath; Cook, Bloomington, IN, USA) was done for vascular plugdeployment; generally, 7 Fr guiding sheath was used. In some cases with very tortuous GR shunt anatomy, two 180 cm 0.035 inch guide wires were used for advancing guiding sheath in the GR shunt. While keeping stable position of guiding sheath, the expect sized vascular plug and 4 Fr angiocatheter were introduced. Amplatzer Vascular Plug II (AGA Medical, Golden Valley, MN, USA) was utilized in all patients. The size of the vascular plug was about 15–25% larger than that suited to the narrowest part of the GR shunt. The retrograde venography was performed using the 4 Fr angiocatheter positioned nearby GV after occluding the GR shunt with the vascular plug. The retrograde venography was usually obtained with contrast hand injection method (3–4 mL/sec, total 9–12 mL). After approximate varix anatomy, including dominant venous tributaries and GR shunt occlusion, was checked with this retrograde venography, the embolization was performed on GV, GR shunt, afferent vein and efferent vein using a gelatin sponge (the size ranged from approximately 1 mm3 to 8 mm3). In two patients having the competitive efferent vein (left inferior phrenic vein), varix embolization was done using gelatin sponge after microcoil embolization to left inferior phrenic vein (Fig. 1). After sufficient gelatin sponge embolization was confirmed by fluoroscopy–i.e., lasting contrast-gelatin sponge mixture stasis in the varix and related venous tributaries and obvious shunt occlusion with vascular plug, 4 Fr angiocatheter was gently moved further downward. When minimal contrast-gelatin sponge mixture oozing into the gastric lumen was noted, more larger size gelatin sponge was used.
The combination of 4 Fr catheter and rapidly infused more larger size gelatin sponge particles was enough to control the oozing varix. The location of the vascular plug and GR shunt occlusion were checked again using the 4 Fr angiocatheter positioned just above vascular plug, the procedure ended with the detachment of the vascular plug.14 In one patient having challenging GR shunt anatomy for guiding sheath advance, CARTO was performed using two microcatheter system [combination of Renegade STC and Renegade Hi-Flo (Boston Sci, Natick, MA, USA)]. A detachable coil embolization (IDC; Boston Sci, Natick, MA, USA) was performed in the GR shunt instead of vascular plug and a gelatin sponge embolization was performed in the GR shunt and GV.15
The immediate vital signs were checked during and after the procedure and closely observed in the intensive care unit until reaching clinical judgment that the patients show no further bleeding sign. The necessity of an additional procedure was determined by evaluating the embolized GV status through a follow-up CT scan 4–5 days after procedure. The general state followed-up including overall liver function tests, was made by observing the outpatient progress thereafter and a GI endoscopy was performed 2–4 months after the procedure. If there were no specific findings, a follow-up CT scan was performed 3–6 months later, and then an endoscopy was performed, if necessary, depending on clinical judgment.
Complete occlusion of GR shunt using a vascular plug or coil with sufficient degree of gelatin sponge embolization of GV, dominant afferent and efferent veins was defined as a technical success, and symptoms resolution related to GV bleeding was defined as a clinical success. Technical success, procedure-related complications, and clinical success were assessed by the primary study end points. Changes in liver function test, changes in existing esophageal varices, occurrence-or-not of new varices and other portal hypertension-related changes before and after the procedure were assessed by the secondary study end points.
Classifications of complication associated with the procedure were classified into major and minor complications according to the clinical practice guidelines of the Society of Interventional Radiology Standards of Practice Committee.21 The laboratory data (international normalized ratio, serum NH3 level, serum total bilirubin level, and serum albumin level) before and after the procedure were compared through the pairedsample t test. Statistical software (SPSS, version 14.0; SPSS Inc., Chicago, IL, USA) was used, and p values less than 0.05 were used for the criteria of the significant difference.
The PARTO procedure was performed on total 8 patients and the CARTO procedure was performed on one patient. GR shunt occlusion was made using one vascular plug [i.e., 12-mm (n=1), 14-mm (n=2), 16-mm (n=4), 18-mm (n=1)] for each patient in the PARTO procedure. A minimal degree of contrast leakage appeared around the GR shunt in one patient while performing the gelatin sponge embolization in the GV and GR shunt after 16-mm vascular plug deployment, therefore, additional GR shunt occlusion procedure was performed using a NBCA glue-lipiodol mixture and two microcoils for enhancing shunt occlusion and prevention of significant complication such as retroperitoneal hemorrhage (Fig. 2). The additional microcoil emblization was performed in the left inferior phrenic vein before vascular plug deployment for two patients (grad 2 and 3 varix anatomy) who showed prominent efferent venous outflow at retrograde venography. A vascular plug deployment was made in tune with the narrowest part of the GR shunt and the subsequent gelatin sponge embolization was made successfully in all patients. There was no immediate complications associated with the PARTO procedure including vascular plug migration. The average time from the selection of GR shunt and placement of vascular plug to detachment was about 37 minutes (25–57 minutes). Total mean procedure time was 68 minutes (48–97 minutes).
The CARTO procedure was performed in one patient; herein, a transjugular approach was attempted at first, but GR shunt angle was not good for the entry of guiding sheath. After switching to a transfemoral approach and then occluding the GR shunt using detachable microcoils (total 16 ea, 14 mm×30 cm to 10 mm×30 cm), the gelatin sponge embolization was performed (Fig. 3).
Complete thromboses of the GV and GR shunt were identified in 7 patients at follow-up CT scan performed on 4–5 days after the procedure. In two patients, focal residual intramural GV–less than 20% of entire pre-procedure GV volume–was noted at short term follow up CT scan, however, these residual varices were obliterated in the 6 months follow-up CT scan.
All patients showed immediate GV bleeding-related symptom resolution and survived until January, 2015, the ending date of the follow-up period of this study and there was no occurrence of bleeding associated with the GV in all the patients over the 17 months (12–32 months), the average duration of the follow up; therefore, the clinical success rate was assessed to be 100%.
Significant variation in liver function test values did not appear before and after the procedure as well as during the follow-up observation period; however, significant decrease of the ammonia level (93 µmol/L → 31 µmol/L, 95% confidence interval: 43 µmol/L, 118 µmol/L; p=0.03) was noted in 5 patients. There was an improvement in clinical symptoms in a grade I hepatic encephalopathy patient.
The follow-up endoscopy was performed 2–4 months later during the follow-up period, and marked shrinkage or disappearance of GV was confirmed in all patients.
The worsening of the existing esophageal varix under the endoscopy was noted in 3 patients (GOV2 n=3/4, 75%), so that an endoscopic variceal ligation was performed prophylactically, even though there was no active esophageal variceal bleeding. Although a small amount of ascites was noted in one patient at short term follow up CT scan, there were no associated clinical symptoms, and ascites disappeared in the 3 months follow up CT.
In this study, we described excellent treatment results of the modified BRTO techniques, such as PARTO and CARTO, confirmed in GV patients under an emergent clinical setting accompanied by acute bleeding. An immediate varix bleeding control could be achieved after the procedure and the procedure could be completed technically in about 1 hour without specific complications. Similar to recently published modified BRTO studies,4581314151622232425 it can be seen that 100% technical and clinical success were achieved.
The use of permanent occlusive devices such as vascular plug and coil instead of the use of the indwelling balloon catheter that has been used in the conventional BRTO procedure made us to obtain a permanent GR shunt occlusion and terminate the procedure in one stage. Thus, the procedure shorted the procedure time and blocked various complications associated with the use of indwelling balloon catheter. There are cases in which repeated procedure was performed in association with recanalized GR shunt and regrowing varix in conventional BRTO-associated studies; the results of this study as well as recently modified BRTO-associated studies led us to conclude that the permanent GR shunt occlusion is effective in fundamentally blocking the residual GV re-growth.1415262728 Even though there was a part of intramural GV left in the short term follow up CT scan in two patients in the present study. However, the entire residual GV was confirmed to have disappeared in the 6 months follow up CT scan. The residual GV in the short term follow up CT scan was in a state of receiving afferent blood flow from the short gastric veins. Nevertheless, it is highly likely that the residual intramural GV was eventually obliterated as the venous flow decreased gradually after complete occlusion of the GR shunt as the dominant outflow route.
Another advantage of this modified technique is that the GV embolization can be performed using a gelatin sponge instead of using various vascular sclerosing agents such as ethanolamine oleate that has been used in the conventional BRTO. PARTO, and also that CARTO can be performed without worrying about the side effects of sclerosing agents previously used–hemolysis, acute renal failure, pulmonary edema, cardiogenic shock, DIC, and anaphylactic reactions.711142225
The technical part considered to demand effort most and technically difficult when performing the modified BRTO procedure is the advance of guiding sheath to an appropriate position for the deployment of a vascular plug. Usually, a 7 Fr guiding sheath-180 cm 0.035 inch guide wire combination was used. However, in some cases, the advance of guiding sheath into sufficient proximal location of GR shunt was facilitated by enhancing the pushability and trackability using the two guide wires. In one patient, a minimal contrast leakage occurred surrounding a GR shunt wall, caused by the pressure rise within the GR shunt during the gelatin sponge embolization of the GR shunt and GV. However, it was treated by using additional embolization materials (NBCA glue-lipiodol mixture and microcoils) without specific complications such as retroperitoneal hemorrhage. Technically, it is expected to restrain the inevitable pressure rise inside the GR shunt by filling the GV with gelatin sponge relatively evenly by positioning the 4 Fr catheter as close to the GV as possible during embolization.
As shown in the earlier BRTO studies, increased portal venous pressure and many consequent clinical conditions associated with portal hypertension, such as deteriorations of accompanied esophageal varices or development of ascites, may appear.1412252930 In the present study, there were worsening esophageal varices in 3 patients out of 4 patients who had existing esophageal varices: preventive endoscopic treatments of the worsened esophageal varices were performed in all three patients during the follow-up observation. However, the modified BRTO treatment itself for acute GV bleeding should be restrained because of potential deterioration of the esophageal varix.
Because of small number of cases in this study, it is hard to generalize. In IGV1 patients group (n=5), no patient showed a newly developed esophageal varix. More research is needed on the portal hemodynamic change between permanent GR shunt occlusion and IGV1. A small amount of ascites appeared transiently in one patient, however, it disappeared, without any medical concern, confirmed in the follow-up.
Many studies found the improvement of liver function over 1–2 months after the BRTO procedure. In this study, however, no statistically significant variation was observed; significant decrease of ammonia level was noted in all 5 patients, and clinical symptom was improved in one patient who had a grade I hepatic encephalopathy. During the follow-up period, there was no significantly meaningful change of hepatic encephalopathy. The usefulness of permanent occlusion of GR shunt for medically uncontrolled hepatic encephalopathy has been investigated by various groups of scientists.124142531
This retrospective study has a few limitations by various groups of scientists. First, we could not conduct a comparative analysis with several GV treatment modalities, including conventional BRTO. In addition, we could not identify the risk of permanent GR shunt occlusion in patients with more complex GR shunt and afferent/efferent venous anatomy because we could not include various anatomical cases that had been proposed in the existing BRTO-associated studies. Prospective, randomized and comparative trials containing much more cases are necessary in future.
In conclusion, the PARTO offers different treatment method that overcomes the limitations encountered in the conventional BRTO and was confirmed to be technically feasible and safe. In addition, it could effectively treat the GV bleeding patients in emergent clinical settings, and excellent treatment performance was confirmed in mid-term clinical follow up as well.
Figures and Tables
Fig. 1
An illustration of vascular plug-assisted retrograde transvenous obliteration (PARTO) procedure. This illustration demonstrates complete obliteration/thrombosis of GV and GR shunt (in gray color). GV gelatin sponge embolization was done via 4 Fr catheter in retrograde fashion. To achieve proper location of vascular plug, guiding sheath advance is most important technical process. GV, gastric varix; GR shunt, gastrorenal shunt.
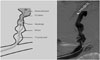
Fig. 2
Fundal GV in a 46-year-old man with massive hematemesis. (A) Contrast-enhanced axial CT images obtained before and after PARTO show completely disappeared fundal GV. (B) Fluoroscopic images of PARTO: minimal contrast leakage after gelatin sponge embolization to massive GR shunt, probably from intra-shunt pressure increase, was controlled by additional embolization. (C) Endoscopic images of the GV also show successful treatment result. Before procedure, massive hemorrhage was noted in the gastric lumen. GV, gastric fundal varix; PARTO, plug assisted retrograde transvenous obliteration; GR, gastrorenal.
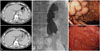
Fig. 3
Fundal GV in a 52-year-old man with massive hematemesis. Coil-assisted RTO (CARTO) was performed after failed guiding sheath advance for vascular plug deployment. Technically successful CARTO demonstrating complete stasis and opacification of GR shunt and GV. GV, gastric fundal varix; RTO, retrograde transvenous obliteration; GR, gastrorenal.

References
1. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001; 5:645–663.

2. Chang CJ, Hou MC, Liao WC, Chen PH, Lin HC, Lee FY, et al. Management of acute gastric varices bleeding. J Chin Med Assoc. 2013; 76:539–546.

3. de Franchis R. Acute variceal haemorrhage: practice guidelines and real-life management. Dig Liver Dis. 2014; 46:398–399.

4. Ryan BM, Stockbrugger RW, Ryan JM. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004; 126:1175–1189.

5. Kiyosue H, Ibukuro K, Maruno M, Tanoue S, Hongo N, Mori H. Multidetector CT anatomy of drainage routes of gastric varices: a pictorial review. Radiographics. 2013; 33:87–100.

6. Kirby JM, Cho KJ, Midia M. Image-guided intervention in management of complications of portal hypertension: more than TIPS for success. Radiographics. 2013; 33:1473–1496.

7. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999; 211:349–356.

8. Park JK, Saab S, Kee ST, Busuttil RW, Kim HJ, Durazo F, et al. Balloon-occluded retrograde transvenous obliteration (BRTO) for treatment of gastric varices: review and meta-analysis. Dig Dis Sci. 2015; 60:1543–1553.

9. Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: part 2. Strategy and techniques based on hemodynamic features. Radiographics. 2003; 23:921–937.
10. Matsumoto A, Hamamoto N, Nomura T, Hongou Y, Arisaka Y, Morikawa H, et al. Balloon-occluded retrograde transvenous obliteration of high risk gastric fundal varices. Am J Gastroenterol. 1999; 94:643–649.

11. Choi SY, Won JY, Kim KA, Lee do Y, Lee KH. Foam sclerotherapy using polidocanol for balloon-occluded retrograde transvenous obliteration (BRTO). Eur Radiol. 2011; 21:122–129.

12. Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012; 29:118–128.

13. Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014; 12:919–928.e1.

14. Gwon DI, Ko GY, Yoon HK, Sung KB, Kim JH, Shin JH, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration--a primary report. Radiology. 2013; 268:281–287.

15. Lee EW, Saab S, Gomes AS, Busuttil R, McWilliams J, Durazo F, et al. Coil-assisted retrograde transvenous obliteration (CARTO) for the treatment of portal hypertensive variceal bleeding: preliminary results. Clin Transl Gastroenterol. 2014; 5:e61.

16. Saad WE, Nicholson DB. Optimizing logistics for balloon-occluded retrograde transvenous obliteration (BRTO) of gastric varices by doing away with the indwelling balloon: concept and techniques. Tech Vasc Interv Radiol. 2013; 16:152–157.

17. Gwon DI, Kim YH, Ko GY, Kim JW, Ko HK, Kim JH, et al. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol. 2015; 26:1589–1595.

18. Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010; 22:1–9.

19. Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: an eleven-year experience. Gastrointest Endosc. 1997; 46:8–14.

20. Sarin SK, Kumar A, Angus PW, Baijal SS, Baik SK, Bayraktar Y, et al. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int. 2011; 5:607–624.

21. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S199–S202.

22. Saad WE, Khaja MS, Hirota S. Balloon-occluded retrograde transvenous obliteration of gastric varices: conception, evolution, and history. Tech Vasc Interv Radiol. 2012; 15:160–164.

23. Sonomura T, Ono W, Sato M, Sahara S, Nakata K, Sanda H, et al. Emergency balloon-occluded retrograde transvenous obliteration of ruptured gastric varices. World J Gastroenterol. 2013; 19:5125–5130.

24. Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996; 167:1317–1320.

25. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996; 11:51–58.

26. Katoh K, Sone M, Hirose A, Inoue Y, Fujino Y, Onodera M. Balloon-occluded retrograde transvenous obliteration for gastric varices: the relationship between the clinical outcome and gastrorenal shunt occlusion. BMC Med Imaging. 2010; 10:2.

27. Kiyosue H, Matsumoto S, Onishi R, Okahara M, Hori Y, Yamada Y, et al. [Balloon-occluded retrograde transvenous obliteration (B-RTO) for gastric varices: therapeutic results and problems]. Nihon Igaku Hoshasen Gakkai Zasshi. 1999; 59:12–19.
28. Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005; 184:1340–1346.

29. Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988; 95:434–440.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download