Abstract
Purpose
The efficacy of helmet continuous positive airway pressure (CPAP) in hypoxemic acute respiratory failure (hARF) remains unclear. The aim of this meta-analysis was to critically review studies that investigated the effect of helmet CPAP on gas exchange, mortality, and intubation rate in comparison with standard oxygen therapy.
Materials and Methods
We performed a meta-analysis of randomized controlled trials (RCTs) by searching the PubMed, Embase, Cochrane library, OVID, and CBM databases, and the bibliographies of the retrieved articles. Studies that enrolled adults with hARF who were treated with helmet CPAP and measured at least one of the following parameters were included: gas exchange, intubation rate, in-hospital mortality rate.
Results
Four studies with 377 subjects met the inclusion criteria and were analyzed. Compared to the standard oxygen therapy, helmet CPAP significantly increased the PaO2/FiO2 [weighted mean difference (WMD)=73.40, 95% confidence interval (95% CI): 43.92 to 102.87, p<0.00001], and decreased the arterial carbon dioxide levels (WMD=-1.92, 95% CI: -3.21 to -0.63, p=0.003), intubation rate [relative risk (RR)=0.21, 95% CI: 0.11 to 0.40, p<0.00001], and in-hospital mortality rate (RR=0.22, 95% CI: 0.09 to 0.50, p=0.0004).
Conclusion
The results of this meta-analysis suggest that helmet CPAP improves oxygenation and reduces mortality and intubation rates in hARF. However, the significant clinical and statistical heterogeneity of the literature implies that large RCTs are needed to determine the role of helmet CPAP in different hypoxemic ARF populations.
Oxygen therapy and endotracheal intubation ventilation are standard medical treatments for hypoxemic acute respiratory failure (hARF).1 Continuous positive airway pressure (CPAP) is a common mode in noninvasive ventilation (NIV) that delivers a constant positive airway pressure during both inspiration and expiration.23 CPAP has been demonstrated to improve oxygen saturation, relieve dyspnea, and avoid complications resulting from intubation, thus playing an important role in treating hARF.45
A recent multicenter randomized controlled trial (RCT) com-pared CPAP delivered by a full-face mask with standard oxygen therapy in patients with hARF, caused by various etiologies.5 The authors observed improved oxygenation during CPAP treatment; however, CPAP did not reduce the rates of intubation complications, in-hospital mortality, and adverse events.
The success of NIV is dictated in part by the choice of interface,6 which greatly affects patient comfort. In Europe, oralnasal masks are the most commonly used interface for NIV;7 however, the helmet interface has gained wide acceptance in some countries, such as Italy, as a more comfortable interface for patients with hARF.7
Several studies have evaluated the efficacy of helmet CPAP for hARF,89101112 and most have found that helmet CPAP significantly reduced the intubation rate and mortality while improving clinical outcomes compared to standard oxygen therapy. However, a robust conclusion has not been reached on the utility of helmet CPAP owing to the small sample size in current RCTs. This problem would remain until a meta-analysis is performed to pool and quantify the results from these studies. For this purpose, the present study was designed and conducted to compare the efficacy of helmet CPAP with standard oxygen therapy in hARF.
According to the guidelines described by the Cochrane handbook for reviews of intervention (version 5.0.1), we searched the PubMed, Embase, Cochrane library, OVID, and CBM databases through March 2015. The literature search was limited to human studies using the following key words: NIV OR noninvasive ventilation OR continuous positive airway pressure OR CPAP OR pressure support ventilation AND acute respiratory failure AND helmet. The reference lists of identified studies were manually reviewed and additional search of related studies were performed if necessary. Two reviewers independently extracted the data using a pre-defined protocol. Any divergence between them was resolved by consensus or by a third reviewer. The following data were recorded from each study: first author, year of publication, study design, trial type, location, primary disease, sample size, experimental and control treatment strategies, and outcome variables.
Studies meeting the following criteria were included: RCT; adult subjects (>18 y); helmet CPAP intervention versus standard oxygen therapy; hARF diagnosed according to a respiratory rate ≥25/min, PaO2 <60 mm Hg, oxygen saturation <90% while breathing room air as measured by pulse oximetry, and PaO2/FIO2 ≤300; hARF secondary to acute exacerbation of chronic obstructive pulmonary disease, pneumonia, cardiogenic pulmonary edema, ALI/ARDS, or similar conditions; measurement of gas exchange, intubation, in-hospital mortality.
Studies were excluded according to the following criteria: subjects <18 years of age; publication as an abstract only; and poor quality (small studies) or sparse data.
The two reviewers independently performed a methodologic quality assessment with divergences resolved by consensus. The risk of bias was assessed using the Cochrane Risk of Bias Tool and evaluated as high, low, or unclear.13
The impact of heterogeneity on the pooled estimates of individual outcomes was assessed using the Cochran Q statistic and I2 test14 to identify inconsistencies between the study results. Inconsistencies were considered as approximately the proportion of total variation in the study estimates that was due to heterogeneity rather than sampling error. Because the Cochran Q test has a low sensitivity for evaluating heterogeneity, a p value of <0.1 was considered significant for the presence of statistical heterogeneity. I2<25%, 25%≤I2<50%, and I2≥50% were considered to indicate low, moderate, and high significance, respectively.15
The statistical analysis was performed using Review Manager (version 5.3.3, The Nordic Cochrane Center, The Cochrane Collaboration; Copenhagen, Denmark). For binary variables in each study, the relative risk (RR) and 95% confidence intervals (95% CI) were calculated, and we computed the weighted mean difference (WMD) and 95% CI for consecutive variables. A fixed effects model was used initially; however, in case of significant heterogeneity across trials, a random effects model was used (p<0.10 and I2>50%).
The database search yielded 308 studies (Fig. 1). Of these, 304 studies were excluded for the following reasons: 145 duplicate studies; 45 case reports and abstracts; 97 studies without examination of hARF; 15 non-RCTs; one incomplete hARF study carried out by Fasano, et al.;16 and one study by Antonaglia, et al.17 using facial NIV in the control group.
The risk of bias in all four trials is shown in Fig. 2. A random number generator was used to generate allocation sequences in all four trials. Appropriate allocation and concealment methods were used in three trials8911 but were not specified in the fourth.17 Blinding was not performed in any trial. Since the appearance of the various NIV masks and treatment devices cannot be identical, the subjects were able to recognize easily the treatment they had received. As a result, trials with adequate randomization and a clear follow-up protocol were considered to be at low risk of bias. According to their random sequence generation, attribution bias, and reporting bias, all trials were at low risk.
All four trials (377 subjects) reported gas exchange data, which are pooled and summarized in Fig. 3 and 4. In patients treated by helmet CPAP, the WMD PaO2/FiO2 was significantly higher at 73.40 (95% CI: 43.92 to 102.87, p<0.00001) (Fig. 3) despite the significant heterogeneity between the trials (I2=93%). Helmet CPAP also decreased the WMD arterial carbon dioxide to -1.92 (95% CI: -3.21 to -0.63, p=0.003) (Fig. 4), compared to patients receiving standard oxygen therapy.
The intubation rate in patients receiving helmet CPAP was significantly lower than that in patients receiving standard oxygen therapy (RR=0.21, 95% CI: 0.11 to 0.40, p<0.00001) (Fig. 5). The in-hospital mortality rate was also reduced in patients treated by helmet CPAP (RR=0.22, 95% CI 0.09 to 0.50, p=0.0004) (Fig. 6).
The present meta-analysis may add to the currently lacking data comparing helmet CPAP and oxygen therapy in hypoxemic acute respiratory failure. Our results suggest that helmet CPAP improves gas exchange and decreases the intubation and in-hospital mortality rates. However, these results are limited by the presence of significant clinical and statistical heterogeneity.
CPAP does not rely on intubation and is still competent to improve the low alveolar ventilation, functional residual capacity, which ultimately relieves dyspnea, ameliorate gas exchange, and reduces the respiratory muscle load.181920 CPAP improves gas exchange ability during hypoxemic ARF caused by various causes.5202122 Similarly, L'Her, et al.23 found that the use of CPAP in patients with acute lung injury, improved oxygenation. Most studies have examined NIV delivered through a face mask or oro-nasal mask. Recent studies suggest differentiating use of CPAP and NIV in ARF according to the specific clinical condition, but strong clinical evidence is still lacking.24 The efficacies of helmet CPAP and facial mask CPAP in hARF patients were evaluated by Tonnelier, et al.,25 who found that both helmet and face mask CPAP increased oxygenation and produced similar outcomes. In the present meta-analysis, we found that helmet CPAP significantly improved gas exchange in patients with hARF, similar to previous reports. However, the statistical heterogeneity was significant between the trials due to the presence of clinical heterogeneity and variations in the timing of blood gas analysis between the primary diseases.
CPAP improves oxygenation and relieves dyspnea, which should decrease the intubation rate and in-hospital mortality rate. CPAP can improve gas exchange ability in patient with ARF, however, robust clinical data indicating that CPAP delivered by facial mask cannot reduce the intubation and mortality rates in hARF in different clinical conditions are lacking.52627 This failure was attributed to the disadvantages of face mask NIV,211 such as intolerance, leakage, and discomfort, which hinder its continuous use.28 As a result, the success of NIV relies on properly establishment of the interface.6 Due to mostly favorable characteristics of the helmet, such as less air leakage and the absence of any contact with the face, the result in the present study indicates fewer complications and better tolerance of the helmet interface compared with a face mask.2125282930 In contrary to the earlier study on facial mask CPAP, the present meta-analysis found that helmet CPAP reduced the intubation and mortality rates significantly.
The present meta-analysis had several limitations. First, the major limitation of the present meta-analysis is the clinical heterogeneity of subjects and the statistical heterogeneity in the included trials. Among the 377 subjects, 128 had moderate-severe acute respiratory failure from pneumonia, whereas 209 had moderate acute respiratory failure in post-operative hypoxemia, and 40 subjects had acute lung injury with hematologic malignancy where pneumonia had been excluded. In addition, none of the included trials were blinded; although the interventions, clinical decisions, and measured variables were prioritized in the literature search, bias remained beyond our control. Second, the number of eligible studies was relatively small, and we found high heterogeneity in their measurement of oxygenation and intolerance between the studies. We attempted to mitigate this heterogeneity by stratifying the studies, based on potentially important variables such as primary disease, hypoxemia severity, and patient age. However, due to the small number of eligible studies, this type of analysis was not feasible or yielded unreliable results. Hence, the results should be interpreted with prudence because they might have been influenced by the paucity and heterogeneity of analyzed data. Additional studies are needed comparing the efficacies of helmet CPAP and face mask CPAP.
In conclusion, the results of this meta-analysis suggest that helmet CPAP improves oxygenation and reduces mortality and intubation rates in hypoxemic acute respiratory failure. However, the significant clinical and statistical heterogeneity of the literature implies that large RCTs are needed to determine the role of helmet CPAP in different hypoxemic ARF populations.
Figures and Tables
Fig. 2
The reviewers made judgments about risk of bias for each item in each included study. +, low risk; ?, unclear risk; -, high risk.
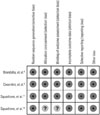
Fig. 3
Forest plot: effect of helmet CPAP on oxygenation (PaO2/FiO2) in patients with hARF. CI, confidence interval; CPAP, continuous positive airway pressure; hARF, hypoxemic acute respiratory failure.

Fig. 4
Forest plot: effect of helmet CPAP on PaCO2 in patients with hARF. CI, confidence interval; CPAP, continuous positive airway pressure; hARF, hypoxemic acute respiratory failure.

Fig. 5
Forest plot: effect of helmet CPAP on intubation in patients with hARF. CI, confidence interval; CPAP, continuous positive airway pressure; hARF, hypoxemic acute respiratory failure.

Fig. 6
Forest plot: effect of helmet CPAP on in-hospital mortality in patients with hARF. CI, confidence interval; CPAP, continuous positive airway pressure; hARF, hypoxemic acute respiratory failure.

Table 1
Main Characteristics of Included Studies

| First author | Year | Location | Study design | Trial type | Inclusion criteria | Experimental strategy | Control strategy | Sample size | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | |||||||||
| Squadrone, et al.11 | 2005 | Italy | RCT | Multicenter | Postoperative patient with acute hypoxemic | Oxygen at FiO2 of 0.5 plus a helmet CPAP of 7.5 cmH2O | Oxygen through a venturi mask at an FiO2 of 0.5 | 105 | 104 | Gas exchange, intubation, in-hospital mortality, adverse events, intolerance |
| Squadrone, et al.10 | 2010 | Italy | RCT | Single-center | Hematologic malignancy with hypoxemic ARF | Oxygen at FiO2 of 0.5 plus a helmet CPAP of 10 cmH2O | Oxygen through a venturi mask at an FiO2 of 0.5 | 20 | 20 | Gas exchange, intubation, in-hospital mortality, adverse events, intolerance |
| Cosentini, et al.9 | 2010 | Italy | RCT | Multicenter | Community-acquired pneumonia with hARF | Helmet CPAP with an initial PEEP of 10 cmH2O and with an FiO2 set to maintian a SpO2≥92% | Oxygen through a venturi mask with an FiO2 set to maintian a SpO2≥92% | 20 | 27 | Gas exchange, intubation, in-hospital mortality, intolerance |
| Brambilla, et al.8 | 2014 | Italy | RCT | Multicenter | Pneumonia with hARF | Helmet CPAP with an initial PEEP of 10 cmH2O and with an FiO2 set to maintian a SpO2≥92% | Oxygen through a venturi mask with an FiO2 set to maintian a SpO2≥92% | 40 | 41 | Gas exchange, intubation, in-hospital mortality, intolerance |
ACKNOWLEDGEMENTS
We are most grateful to Guangqiao Zeng, MD, State Key Laboratory of Respiratory Disease, Guangzhou Medical University, Guangzhou, China, for his assistances in medical writing. This work was supported by the Guangdong Provincial Science and Technology Project (2013B022000072).
Yuwen Luo was involved in the conception and design of the study, analysis of the data and drafting of the article. Xin Chen was involved in analysis of the data and revision of the manuscript. Yan Luo was involved in the conception of the study and drafting of the article. Yun Li, Zhe Zhu, Luqian Zhou, Yitai Chen, and Yuxia Huang were involved in the design of the study, drafting of the article and critical revision of the manuscript. All authors approved the final version of the manuscript.
References
1. Esquinas Rodriguez AM, Papadakos PJ, Carron M, Cosentini R, Chiumello D. Clinical review: helmet and non-invasive mechanical ventilation in critically ill patients. Crit Care. 2013; 17:223.

4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014; 9:837–852.
5. Delclaux C, L'Her E, Alberti C, Mancebo J, Abroug F, Conti G, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000; 284:2352–2360.

6. Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009; 54:71–84.
7. Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010; 36:362–369.

8. Brambilla AM, Aliberti S, Prina E, Nicoli F, Del Forno M, Nava S, et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014; 40:942–949.

9. Cosentini R, Brambilla AM, Aliberti S, Bignamini A, Nava S, Maffei A, et al. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010; 138:114–120.

10. Squadrone V, Massaia M, Bruno B, Marmont F, Falda M, Bagna C, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. 2010; 36:1666–1674.

11. Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005; 293:589–595.

12. Cammarota G, Vaschetto R, Turucz E, Dellapiazza F, Colombo D, Blando C, et al. Influence of lung collapse distribution on the physiologic response to recruitment maneuvers during noninvasive continuous positive airway pressure. Intensive Care Med. 2011; 37:1095–1102.

13. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.

14. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997; 127:820–826.

15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

16. Fasano L, Mega C, Pisani L, Navalesi P, Bellone A, Scala R, et al. Efficacy of helmet as interface for noninvasive ventilation (NIV) in acute hypercapnic respiratory failure (AHRF). Chest. 2012; 142(4_MeetingAbstracts):946A.

17. Antonaglia V, Ferluga M, Molino R, Lucangelo U, Peratoner A, Roman-Pognuz E, et al. Comparison of noninvasive ventilation by sequential use of mask and helmet versus mask in acute exacerbation of chronic obstructive pulmonary disease: a preliminary study. Respiration. 2011; 82:148–154.

18. Chiumello D, Esquinas AM, Moerer O, Terzi N. A systematic technical review of the systems for the continuous positive airway pressure. Minerva Anestesiol. 2012; 78:1385–1393.
19. Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009; 374:250–259.

20. Lindner KH, Lotz P, Ahnefeld FW. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987; 92:66–70.

21. Principi T, Pantanetti S, Catani F, Elisei D, Gabbanelli V, Pelaia P, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med. 2004; 30:147–150.

22. Brett A, Sinclair DG. Use of continuous positive airway pressure in the management of community acquired pneumonia. Thorax. 1993; 48:1280–1281.

23. L'Her E, Deye N, Lellouche F, Taille S, Demoule A, Fraticelli A, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005; 172:1112–1118.
24. Nava S, Carlucci A. Non-invasive pressure support ventilation in acute hypoxemic respiratory failure: common strategy for different pathologies? Intensive Care Med. 2002; 28:1205–1207.

25. Tonnelier JM, Prat G, Nowak E, Goetghebeur D, Renault A, Boles JM, et al. Noninvasive continuous positive airway pressure ventilation using a new helmet interface: a case-control prospective pilot study. Intensive Care Med. 2003; 29:2077–2080.

26. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998; 114:1185–1192.

27. Keenan SP. Noninvasive positive pressure ventilation in acute respiratory failure. JAMA. 2000; 284:2376–2378.

28. Chiumello D, Pelosi P, Carlesso E, Severgnini P, Aspesi M, Gamberoni C, et al. Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med. 2003; 29:1671–1679.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download