Abstract
Purpose
The purpose of this study was to define the role of cyclooxygenase-2 inhibitors (COX-2i) in reducing hepatic fibrosis in pediatric patients with chronic liver disease.
Materials and Methods
From September 2009 to September 2010, patients over 2 years old who visited our outpatient clinic for follow-up to manage their chronic liver disease after Kasai portoenterostomy for biliary atresia, were included in this study. Volunteers were assigned to the study or control groups, according to their preference. A COX-2i was given to only the study group after obtaining consent. The degree of hepatic fibrosis (liver stiffness score, LSS) was prospectively measured using FibroScan, and liver function was examined using serum analysis before and after treatment. After 1 year, changes in LSSs and liver function were compared between the two groups.
Results
Twenty-five patients (18 females and 7 males) were enrolled in the study group. The control group included 44 patients (26 females and 18 males). After 1 year, the least square mean values for the LSSs were significantly decreased by 3.91±0.98 kPa (p=0.004) only in the study group. Serum total bilirubin did not decrease significantly in either group.
Most patients who undergo a Kasai portoenterostomy for biliary atresia (BA) develop chronic liver disease.1 Cholangitis, portal hypertension, variceal bleeding, liver cirrhosis, ascites, or splenomegaly commonly occur after a Kasai portoenterostomy for BA.123 Hepatocellular carcinoma (HCC) that complicates biliary cirrhosis has also been reported in patients with BA.45
In a recent report, increased expression of cyclooxygenase-2 (COX-2) in biliary epithelial cells was found in BA patients with severe liver dysfunction at the time of the Kasai procedure.6 Overexpression of COX-2 has been demonstrated in inflammatory, cirrhotic, or cancerous liver tissue,789 and many studies have tried to elucidate the role of COX-2 inhibitors (COX-2i) in hepatitis, portal hypertension, liver cirrhosis, and hepatic carcinogenesis using animal models.1011121314151617 Among these studies, we previously reported the effect of a selective COX-2i in reducing hepatic fibrosis in rats with ligated common bile ducts.14
The purpose of our current study was to evaluate the effect of a COX-2i in ameliorating hepatic fibrosis or chronic inflammation in children with chronic liver disease.
The study protocol was explained to all patients (and their parents) who were older than 2 years of age and who reported to our outpatient clinic for management of their BA after Kasai procedures. Patients, enrolled in the study and control groups, provided written informed consent, and they volunteered for the study or control group. Because the safety of the drug (meloxicam) used in the study group was not approved in children younger than 2 years of age, patients younger than 2 years were excluded from both groups.
This clinical trial was registered at www.clinicaltrials.gov (trial identification number: NCT02298218).
From September 2009 to September 2010, patient volunteers who were over 2 years old, who had undergone a Kasai portoenterostomy for BA, and who were postoperatively managed in our outpatient clinic were prospectively enrolled in this study. The study was approved by the Ethics Committee of Severance Hospital (approval number 4-2008-0597). Serum and urine analysis, abdominal ultrasonography, and a stool occult blood test were performed as screening tests to determine hepatic and renal function and to exclude patients with hematologic diseases, varices, or ulcers. Endoscopy of the upper gastrointestinal tract was not routinely performed as a screening test because of the invasiveness of endoscopy. If the stool occult blood test was positive, an endoscopy was performed to check for esophageal or gastric varices or ulcers. Patients with any hepatic, renal, or hematologic abnormalities or abnormal endoscopic findings were excluded. For each patient, hepatic abnormalities identified in the screening test were considered to be markedly outstanding results from the range of their previous data of serum analysis or sonographic findings.
Although patients were originally grouped into high-dose or low-dose groups using a randomized sampling method, no important differences were found. Therefore, the data were combined for all analyses. A selective COX-2i, meloxicam (Melax, Chong Kun Dang Pharm., Seoul, Korea) was prescribed to the patients once daily according to their grouping: 0.0625 mg/kg body weight in the low-dose group and 0.125 mg/kg in the high-dose group.
The degree of hepatic fibrosis was determined by the liver stiffness score (LSS) using a FibroScan (Echosens, Paris, France). The LSS was determined three times: baseline, 6 months, and 1 year after enrollment in the study group. At the 6-month time point, if the score was elevated more than 10% compared to the baseline value, the patient was excluded from the study group. If the change in the LSS at 6 months was less than or equal to 110%, the study drug was maintained for another 6 months. The LSS was determined at the 1-year time point, which corresponded to the time of study completion. Liver function was checked by serum analysis before and after receiving the COX-2i.
Patients visited the outpatient clinic every 3 months to check for any adverse effects of the drug. Furthermore, serum analysis, including a blood cell count, analysis of hepatic and renal function, urinalysis, abdominal ultrasonography, and a stool occult blood test were performed every 3 months. The trough level of the drug in the serum was checked to maintain the concentration of the drug in the proper range. If any suspicious adverse effect of the drug was noted, the patient was excluded from the study.
The LSS has been determined in patients with BA every year at our outpatient clinic since the introduction of the FibroScan in 2007. The control group included patients who were followed at our outpatient clinic after successful Kasai portoenterostomy for BA and who simultaneously had their LSS and serum total bilirubin measured two or more times during the post-operative follow-up period. LSS was measured two times every 6 months after enrollment in the control group. We excluded the LSS from our analysis of patients in the control group when the LSS was measured with a success rate lower than 60%, when patients had hyperbilirubinemia (elevation of serum total bilirubin ≥1.0 mg/dL compared to the baseline), after a repeat-Kasai procedure, or after liver transplantation. Except for medication with the COX-2i in the study group, all management procedures after the Kasai portoenterostomy were the same in both groups, including administration of ursodeoxycholic acid, antibiotics to prevent cholangitis, and the use of transient steroid therapy.
LSS was measured in both groups as described previously.18 In brief, the patient was placed in a supine position with maximal abduction of the right arm. Ten successful measurements of LSS were obtained with an ultrasound transduced probe on the right lobe of the liver through the intercostal spaces. The median value of the 10 measurements of LSS was calculated automatically by software in FibroScan and expressed as LSS in kilopascals (kPa).
The LSS and serum total bilirubin values in both groups were collected retrospectively for a period of 2 years before enrollment in the study, and they were compared with those values after study enrollment. The median LSS value and the average of the serum total bilirubin for each patient were used as the baseline values.
Statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA) and SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA). Categorical parameters were analyzed with Pearson's chi-squared test. Continuous parametric data were analyzed with independent t test. Paired non-parametric data were analyzed with Wilcoxon signed-rank test. The effects of the study drug on LSS, serum bilirubin, and liver enzymes were compared between the two groups using a linear mixed model. P values for multiple comparisons were adjusted with post-hoc analysis using Bonferroni's correction. A p value less than 0.05 was considered statistically significant.
The clinical characteristics of both groups are presented in Table 1. Twenty-five patients were enrolled in the study group (18 females and 7 males) with an average age at enrollment of 6.1±3.0 years. The control group included 44 patients (26 females and 18 males) with an average age at enrollment of 5.5±3.2 years. The mean age at the time of Kasai portoenterostomy for BA was not significantly different between the two groups (66.3±36.6 days in the study group vs. 58.0±25.3 days in the control group; p=0.279). Thirteen patients (10 females and 3 males) were assigned randomly to the low-dose group prescribed 0.0625 mg/kg body weight COX-2i, and 12 patients (8 females and 4 males) were assigned to the high-dose group prescribed 0.125 mg/kg COX-2i.
The average value for serum total bilirubin before study enrollment was not different between the two groups (1.4±0.7 mg/dL in the study group vs. 0.9±1.5 mg/dL in the control group; p=0.175). However, the mean LSS before study enrollment was higher in the study group than in the control group (20.5±9.7 kPa in the study group vs. 11.8±8.9 kPa in the control group; p<0.001) (Table 1). The success rate of obtaining LSS before study enrollment was more than 90% in both groups (96.7±9.0% in the study group vs. 93.2±10.7% in the control group; p=0.153). During the study, no LSS data were excluded because all measured success rates of LSS were higher than 80% in both groups.
The least square means (LSM) of the LSS in the study group were significantly decreased by 3.91±0.98 kPa at the 1-year time point. In contrast, the LSS in the control group did not change significantly during the study period with a difference of -0.29±0.70 kPa (p=0.004) (Fig. 1A). Serum total bilirubin levels did not change significantly in either group during the study period (Fig. 1B). Differences of the LSM of serum total bilirubin during the 1-year study period were -0.34±0.17 mg/dL in the study group and 0.02±0.08 mg/dL in the control group (p=0.071) (Table 2). The liver enzymes [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] in either group did not change significantly during the study period (Table 2, Fig. 1C and D).
During the study period, the mean serum drug level in the study group was 0.27±0.17 µg/mL. Considering the possible side effects of COX-2i, we performed serum analysis including a blood cell count, hepatic and renal function tests, urinalysis, abdominal ultrasonography, and stool occult blood test every 3 months. LSS was measured 6 months after the start of medication. If the LSS was elevated by more than 10% at the 6-month time point compared to baseline, or if an abnormal finding showed up in the follow-up tests every 3 months, we excluded the patient from the study group to prevent the possible occurrence of hepatic toxicity or other adverse effect of the drug. Three patients in the study group (two in the high-dose group and one in the low-dose group) were excluded at the 6-month time point, because their LSS were elevated by more than 10% compared to baseline. However, the median values of their LSSs were not significantly different with 12.60 (7.75–27.90) kPa at baseline LSS and 13.50 (9.50–45.00) kPa at the 6-month time point (p=0.109). Moreover, serum total bilirubin and liver enzymes were unchanged at the 6-month time point (p=0.102 for serum total bilirubin, p=0.102 for AST, and p=0.109 for ALT). Their elevated LSSs and the other data obtained at the 6-month time point were included in the data analysis as the study group without the data at the 12-month time point. Moreover, no adverse effects due to COX-2i were noted in this study. The results of the serum analysis and the urinalysis indicated that hematologic, hepatic, and renal function values were not affected by the use of COX-2i during the study. The occult blood test result was negative for every stool test. When they were asked during follow-up visits, none of the patients complained of nausea, vomiting, diarrhea, bloody stool, indigestion, abdominal pain, heartburn, chest discomfort or tightness, mood change, anxiety, change in appetite, skin rash, irritability, abnormal sensation, changes in vision, confusion, or loss of consciousness.
Despite the overall success of the Kasai procedure for BA,1920 many children with BA experience liver damage after the procedure.2 This liver damage, which includes fibrosis and cholangitis, can lead to chronic liver disease with portal hypertension, cirrhosis, or even HCC and the need for a liver transplant.23452122 Although approximately 80–90% of patients who undergo the Kasai procedure or even liver transplantation survive to adolescence and beyond,2320 these patients experience substantial morbidity. Liver fibrosis and cirrhosis begin early in infants with BA.2 Corticosteroids may help limit inflammatory damage and increase bile flow, but their efficacy remains unclear.23 Therefore, additional pharmacological treatments are required to improve liver function in these patients.
Overexpression of COX-2 in the liver has been observed in patients with chronic hepatitis, cirrhosis, and HCC,7891516 and COX-2 may mediate or worsen these conditions.67915 Liver fibrosis is caused by cholestasis and collagen accumulation, and COX-2 is upregulated with these conditions.13 COX-2 expression also correlates with the stage of fibrosis.9 Mohammed, et al.7 analyzed COX-2 expression in cirrhotic livers after hepatitis B and C infection and found that COX-2 was absent in normal livers but high in cirrhotic livers. Jeong, et al.23 examined COX-2 protein expression in 43 patients with chronic hepatitis and 24 patients with cirrhosis using immunohistochemistry and found that COX-2 expression was higher in patients with cirrhosis and advanced fibrosis. Honsawek, et al.6 reported that COX-2 expression was increased in biliary epithelial cells at the time of Kasai portoenterostomy in patients with BA and was associated with an adverse postoperative outcome.
In addition, COX-2 may be involved in the development of HCC resulting from chronic liver disease. In patients with HCC and hepatitis C virus-related cirrhosis, overexpression of COX-2 may accelerate the development of HCC, and higher COX-2 expression is a significant risk factor for recurrence of HCC in the residual liver.8 He, et al.24 evaluated COX-2 expression in the liver of patients with HCC and showed that the recurrence-free survival rates in the COX-2-positive group were significantly lower than those in the COX-2-negative group. These authors speculated that overexpression of COX-2 in the noncancerous liver may predict cancer recurrence in patients with hepatitis B virus-related cirrhosis.24 Thus, blocking COX-2 activity may improve liver function and prevent further damage in different groups of patients, including those with BA.
The effect of inhibiting COX-2 has been explored in several animal models of liver disease. In rats treated with carbon tetrachloride to induce liver cirrhosis, the COX-2i rofecoxib reduces portal pressure, collagen accumulation, and fibrogenesis.11 Using a similar rat model, Chávez, et al.12 showed that the COX-2i celecoxib prevents and reduces cholestatic damage and collagen deposition, demonstrating the antifibrogenic and fibrolytic effects of this COX-2i. Similarly, carbon tetrachloride-treated mice given the COX-2i SC-236 show reduced liver fibrosis.13 In an animal study,14 we performed bile duct ligation in rats to induce cholestasis, which triggers fibrogenesis in the liver, and subsequently treated the rats with the COX-2i meloxicam, and found that meloxicam reduces fibrosis and collagen accumulation, with corresponding histological improvement of the liver by attenuating the expression of α-smooth muscle actin, transforming growth factor-β1, COX-2, and matrix metalloproteinase-9. The preventive effect of meloxicam was also reported in the rat hepatic ischemia/reperfusion injury model.15 Yamamoto, et al.10 showed that the COX-2i JTE-522 prevents liver fibrosis in rats that were fed a choline- and L-amino acid-deficient diet or thioacetamide to induce fibrosis. Thus, inhibiting COX-2 seems to prevent many different types of liver damage from a variety of causes in animals. In a recent report, Edfawy, et al.16 suggested that the hepatoprotective effect of COX-2i could be ascribed to its antioxidant potential, free radical scavenging properties, anti-inflammatory effect, and ability to abrogate apoptosis via suppression of active caspase 3. In another study, selective COX-2i played a role in decreasing portal hypertension via its dual inhibitory effects on intrahepatic fibrosis and angiogenesis.17 The antifibrotic effects of COX-2i have been shown in models of fibrosis in other organs including the heart,25 lung,26 kidney,2728 and peritoneum.29 To our knowledge, however, no study of human subjects has found that COX-2i can prevent liver damage; the present study is the first to find an ameliorating effect of COX-2i in humans with chronic liver disease.
In this study, the study group initially had a higher mean LSS, which indicates more advanced liver disease compared to the control group. This difference in patient groups might have been caused by the voluntary grouping of the patients. Sicker patients may want another option to manage their chronic liver disease. However, after medication with COX-2i, most patients in the study group had improved LSS. Although LSS decreased over time in both groups, it was significantly decreased only in the study group. Thus, treatment with COX-2i can reduce the LSS in patients with chronic liver disease after Kasai portoenterostomy for BA.
Meloxicam is a non-steroidal anti-inflammatory drug used to treat pain and inflammation caused by osteoarthritis or rheumatoid arthritis in adults and children who are at least 2 years old. The usual pediatric dosage for juvenile rheumatoid arthritis is 0.125 mg/kg/day, which was the dosage in our high-dose group. The maximal dose for juvenile rheumatoid arthritis is 7.5 mg/day. No dosage adjustment is needed in patients with mild to moderate hepatic or renal insufficiency.30 Reduction of the LSS was achieved in our study group using the universal dosage for juvenile rheumatoid arthritis or even 50% of that dose. No adverse reaction related to the COX-2i was found after the 1-year study period. The LSS in the study group was significantly decreased only after administration for 1 year (not for 6 months) compared to the control group. Therefore, we cautiously suggest that long-term treatment for 1 year may be necessary to reduce the LSS without drug side effects in pediatric patients with chronic liver disease.
At the midpoint of the study, we excluded three patients from the study group because of their elevated LSS to prevent possible hepatic adverse effects of the study drug. Doing so may have provided a potential bias in analyzing the result by comparing the study and control groups. However, two of the three excluded patients had a lower LSS than the mean LSS of the study group. Therefore, the bias from the exclusion of these three patients may not be significant.
The LSS is known to be affected by liver fibrosis, cholestasis (which is correlated with serum bilirubin), and necroinflammation of the liver (which is represented by elevated liver enzymes, AST or ALT).313233343536 In our study, treatment with a COX-2i improved the LSS in cholestatic patients with BA who had undergone a Kasai portoenterostomy; however, cholestasis, as measured by total bilirubin, and necroinflammatory activity of the liver, as measured by ALT or AST, were not changed. Therefore, the decrease in LSS indicates that the COX-2i ameliorated liver fibrosis.
In conclusion, COX-2i has a beneficial effect on hepatic fibrosis in children with chronic liver disease after Kasai portoenterostomy for BA. Accordingly, COX-2i may have a preventive effect against hepatic fibrosis in our study group, considering the progression of hepatic fibrosis in the patients with BA even after Kasai portoenterostomy. COX2i may be a promising new treatment for preventing or reducing hepatic fibrosis after Kasai portoenterostomy for BA. Long-term treatment with a universal dose of a COX-2i was safe and did not lead to complications in these young patients with chronic liver disease. Further studies are needed to determine the optimal duration and dosage of the COX-2i in children with chronic liver disease and the optimal time to begin therapy with a COX-2i after the initial Kasai procedure.
Figures and Tables
 | Fig. 1Changes in liver parameters according to the study time points for the study and control groups. (A) Median values of the liver stiffness score (LSS). (B) Mean values of serum total bilirubin (TB). (C) Mean values of aspartate aminotransferase (AST). (D) Mean values of alanine aminotransferase (ALT). The significant difference (p<0.05) is indicated with an asterisk. |
Table 1
Clinical Characteristics of the Study Population
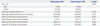
Table 2
The Differences of Least Square Mean Values of the LSS, TB, and Liver Enzyme Values at Baseline, 6-Month, and 1-Year Time Points

ACKNOWLEDGEMENTS
This research was supported by a grant from Yonsei University College of Medicine (grant number 6-2008-0296).
References
1. Hadzić N, Davenport M, Tizzard S, Singer J, Howard ER, Mieli-Vergani G. Long-term survival following Kasai portoenterostomy: is chronic liver disease inevitable? J Pediatr Gastroenterol Nutr. 2003; 37:430–433.

4. Hadžić N, Quaglia A, Portmann B, Paramalingam S, Heaton ND, Rela M, et al. Hepatocellular carcinoma in biliary atresia: King's College Hospital experience. J Pediatr. 2011; 159:617–622.e1.

5. Hol L, van den Bos IC, Hussain SM, Zondervan PE, de Man RA. Hepatocellular carcinoma complicating biliary atresia after Kasai portoenterostomy. Eur J Gastroenterol Hepatol. 2008; 20:227–231.

6. Honsawek S, Klaikeaw N, Vejchapipat P, Chongsrisawat V, Ruangvejvorachai P, Poovorawan Y. Cyclooxygenase-2 overexpression is associated with clinical outcome in biliary atresia. Eur J Pediatr Surg. 2010; 20:164–168.

7. Mohammed NA, Abd El-Aleem SA, El-Hafiz HA, McMahon RF. Distribution of constitutive (COX-1) and inducible (COX-2) cyclooxygenase in postviral human liver cirrhosis: a possible role for COX-2 in the pathogenesis of liver cirrhosis. J Clin Pathol. 2004; 57:350–354.

8. Morinaga S, Tarao K, Yamamoto Y, Nakamura Y, Rino Y, Miyakawa K, et al. Overexpressed cyclo-oxygenase-2 in the background liver is associated with the clinical course of hepatitis C virus-related cirrhosis patients after curative surgery for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007; 22:1249–1255.

9. Pazirandeh S, Khettry U, Gordon FD, Resnick RH, Murray JE, Sheth SG. Cyclooxygenase-2 expression in hepatocellular carcinoma, cirrhosis and chronic hepatitis in the United States. Dig Dis Sci. 2007; 52:220–227.

10. Yamamoto H, Kondo M, Nakamori S, Nagano H, Wakasa K, Sugita Y, et al. JTE-522, a cyclooxygenase-2 inhibitor, is an effective chemopreventive agent against rat experimental liver fibrosis. Gastroenterology. 2003; 125:556–571.

11. Tu CT, Guo JS, Wang M, Wang JY. Antifibrotic activity of rofecoxib in vivo is associated with reduced portal hypertension in rats with carbon tetrachloride-induced liver injury. J Gastroenterol Hepatol. 2007; 22:877–884.

12. Chávez E, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Castro-Sánchez L, et al. Antifibrotic and fibrolytic properties of celecoxib in liver damage induced by carbon tetrachloride in the rat. Liver Int. 2010; 30:969–978.

13. Horrillo R, Planagumà A, González-Périz A, Ferré N, Titos E, Miquel R, et al. Comparative protection against liver inflammation and fibrosis by a selective cyclooxygenase-2 inhibitor and a nonredox-type 5-lipoxygenase inhibitor. J Pharmacol Exp Ther. 2007; 323:778–786.

14. Kim SM, Park KC, Kim HG, Han SJ. Effect of selective cyclooxygenase-2 inhibitor meloxicam on liver fibrosis in rats with ligated common bile ducts. Hepatol Res. 2008; 38:800–809.

15. Tolba RH, Fet N, Yonezawa K, Taura K, Nakajima A, Hata K, et al. Role of preferential cyclooxygenase-2 inhibition by meloxicam in ischemia/reperfusion injury of the rat liver. Eur Surg Res. 2014; 53:11–24.

16. Edfawy M, Hassan MH, Mansour A, Hamed AA, Amin HA. Meloxicam modulates oxidative stress status, inhibits prostaglandin E2, and abrogates apoptosis in carbon tetrachloride-induced rat hepatic injury. Int J Toxicol. 2012; 31:276–286.

17. Gao JH, Wen SL, Yang WJ, Lu YY, Tong H, Huang ZY, et al. Celecoxib ameliorates portal hypertension of the cirrhotic rats through the dual inhibitory effects on the intrahepatic fibrosis and angiogenesis. PLoS One. 2013; 8:e69309.

18. Chang HK, Park YJ, Koh H, Kim SM, Chung KS, Oh JT, et al. Hepatic fibrosis scan for liver stiffness score measurement: a useful preendoscopic screening test for the detection of varices in postoperative patients with biliary atresia. J Pediatr Gastroenterol Nutr. 2009; 49:323–328.

19. Shinkai M, Ohhama Y, Take H, Kitagawa N, Kudo H, Mochizuki K, et al. Long-term outcome of children with biliary atresia who were not transplanted after the Kasai operation: >20-year experience at a children's hospital. J Pediatr Gastroenterol Nutr. 2009; 48:443–450.

20. Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013; 58:1209–1217.

21. Kumagi T, Drenth JP, Guttman O, Ng V, Lilly L, Therapondos G, et al. Biliary atresia and survival into adulthood without transplantation: a collaborative multicentre clinic review. Liver Int. 2012; 32:510–518.

22. Ng VL, Haber BH, Magee JC, Miethke A, Murray KF, Michail S, et al. Medical status of 219 children with biliary atresia surviving long-term with their native livers: results from a North American multicenter consortium. J Pediatr. 2014; 165:539–546.e2.
23. Jeong SW, Jang JY, Lee SH, Kim SG, Cheon YK, Kim YS, et al. Increased expression of cyclooxygenase-2 is associated with the progression to cirrhosis. Korean J Intern Med. 2010; 25:364–371.

24. He YF, Jin J, Wei W, Chang Y, Hu B, Ji CS, et al. Overexpression of cyclooxygenase-2 in noncancerous liver tissue increases the postoperative recurrence of hepatocellular carcinoma in patients with hepatitis B virus-related cirrhosis. Can J Gastroenterol. 2010; 24:435–440.

25. Wang BH, Bertucci MC, Ma JY, Adrahtas A, Cheung RY, Krum H. Celecoxib, but not rofecoxib or naproxen, attenuates cardiac hypertrophy and fibrosis induced in vitro by angiotensin and aldosterone. Clin Exp Pharmacol Physiol. 2010; 37:912–918.

26. Arafa HM, Abdel-Wahab MH, El-Shafeey MF, Badary OA, Hamada FM. Anti-fibrotic effect of meloxicam in a murine lung fibrosis model. Eur J Pharmacol. 2007; 564:181–189.

28. Kucuk HF, Bingul SM, Kurt N, Kaptanoglu L, Akyol H, Torlak OA, et al. Effect of a selective cyclooxygenase-2 inhibitor on renal scarring. Eur Surg Res. 2006; 38:451–457.

29. Fabbrini P, Schilte MN, Zareie M, ter Wee PM, Keuning ED, Beelen RH, et al. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol Dial Transplant. 2009; 24:3669–3676.

30. Drugs.com. Meloxicam. accessed on 2008 November 16. Available at: http://www.drugs.com/meloxicam.html.
31. Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008; 48:1718–1723.

32. Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007; 56:968–973.

33. Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007; 14:360–369.

34. Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008; 47:592–595.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download