Abstract
Purpose
Diagnosis of extrapulmonary tuberculosis (EPTB) poses serious challenges. A careful selection of appropriate gene targets is essential for designing a multiplex-polymerase chain reaction (M-PCR) assay.
Materials and Methods
We compared several gene targets of Mycobacterium tuberculosis, including IS6110, devR, and genes encoding MPB-64 (mpb64), 38kDa (pstS1), 65kDa (hsp65), 30kDa (fbpB), ESAT-6 (esat6), and CFP-10 (cfp10) proteins, using PCR assays on 105 EPTB specimens. From these data, we chose the two best gene targets to design an M-PCR.
Results
Among all gene targets tested, mpb64 showed the highest sensitivity (84% in confirmed cases and 77.5% in clinically suspected cases), followed by IS6110, hsp65, 38kDa, 30kDa, esat6, cfp10, and devR. We used mpb64+IS6110 for designing an M-PCR assay. Our M-PCR assay demonstrated a high sensitivity of 96% in confirmed EPTB cases and 88.75% in clinically suspected EPTB cases with a high specificity of 100%, taking clinical diagnosis as the gold standard.
Tuberculosis (TB) remains one of the foremost infectious diseases throughout the world, with an estimated 9.0 million incident cases in 2013, and India ranks first (24% of cases) in total TB incident cases throughout the world.1 According to the National Tuberculosis Control Programme, 2.6 million new cases of sputum smear-positive pulmonary TB (PTB), 2.0 million new cases of sputum smear-negative PTB, and 0.8 million new cases of extrapulmonary tuberculosis (EPTB) were observed in 2013 worldwide.1 EPTB comprises about 15-20% of TB cases and can comprise up to 50% of TB cases in human immunodeficiency virus (HIV)-infected individuals.12 According to the Revised National Tuberculosis Control Programme, 0.22 million new cases of EPTB were documented in 2013 in India.3 Beyond the Indian subcontinent, EPTB remains a significant health problem in other developing and under-developed countries such as China, Korea, Vietnam, Brazil, Tunisia, Burkina Faso, etc.1
Diagnosis of smear-positive PTB has been somewhat established; however, the diagnosis of smear-negative PTB, TB-HIV co-infection, and EPTB exhibits serious challenges.45 Diagnosis of EPTB can be elusive due to paucibacillary load in the biological specimens, lack of adequate clinical sample volume, and localization of disease at sites that are difficult to access.26 Smear microscopy is the most extensively used method for the diagnosis of EPTB as it is inexpensive and rapid; however, the method has drawbacks due to low and variable sensitivity values (0-40%).7 Culture identification, though considered as a gold standard, has drawbacks including low and variable sensitivities (0-80%) in various clinical forms of EPTB,248 a slow turnaround time, and a labor-intensive process. The BACTEC system, histopathological/cytological examination, and interferon-γ release assays are also employed for the diagnosis of EPTB; however, these assays have their own limitations.27 Detection of adenine deaminase is utilized in diagnosing EPTB specimens, although there is no established cut-off value, and this test has been shown to be positive in other diseases such as lymphomas and collagen vascular diseases.29 Recently, nucleic acid amplification (NAA) tests such as PCR have emerged as potentially important tools for the diagnosis of EPTB specimens. Various mycobacterial gene targets such as insertion sequence (IS)6110; IS1081; 16S rRNA DNA; devR (transcriptional regulator, Rv3133c); genes encoding 65kDa (heat shock protein 65, hsp65; Rv0440), MPB-64/MPT-64 (mycobacterial protein from species tuberculosis, mpb64; Rv1980c), 38kDa (phosphate-binding lipoprotein, pstS1; Rv0934), and MTP-40 (membrane associated phospolipase C1; Rv2351c) proteins; TRC4 (conserved repetitive element); GCRS (guanine-cytosine-rich repetitive sequence); fbp, encoding fibronectin-binding protein B (30kDa, Ag85B protein; Rv1886c); hupB, encoding histone-like DNA-binding protein (Rv2986c); and dnaJ, encoding chaperone protein (Rv0352), have been exploited for such tests.2571011 However, limited information is available for the utilization of the M. tuberculosis-specific region of difference (RD) 1 esat6, encoding early secretory antigenic target-6 (ESAT-6, Rv3875), and cfp10, encoding culture filtrate protein-10 (CFP-10, Rv3874), gene targets. Moreover, there is a high variation in these tests, owing to different gene targets employed as well as the different gold standards adopted in various laboratories. Recently, the introduction of the MTB/RIF GeneXpert (Cepheid, Sunnyvale, CA, USA) assay, which targets conserved rpoB gene encoding RNA β polymerase subunit, has been a major breakthrough in the diagnosis of EPTB;12 however, its use is limited in resource-poor settings due to its high cost. Therefore, we planned a study to compare several gene targets (IS6110, devR, mpb64, hsp65, 30kDa, 38kDa, esat6, and cfp10) for PCR assays on EPTB specimens to identify the two best gene targets exhibiting high sensitivities, using clinical diagnosis as the gold standard, and to design a simple, reliable, and cost-effective multiplex-polymerase chain reaction (M-PCR) assay with the two resulting targets for an early diagnosis of EPTB.
The clinical specimens were collected from the patients visiting the clinical departments of Rajan Babu Institute for Pulmonary Medicine & Tuberculosis, Delhi; Vallabhbhai Patel Chest Institute, University of Delhi; and the Postgraduate Institute of Medical Sciences, Rohtak during the period of June, 2013 to September, 2014. Written informed consent was obtained from all patients participating in this study. This work was part of a project approved by the Maharshi Dayanand University Institute Ethical Committee under the protocol number MDU/CBT/11/793. This study was double blinded, as the laboratory was not aware of the clinical data and the clinicians were not aware of the laboratory data until all of the analyses were complete.
The sample size (n=155) was determined to be statistically significant with a 95% level of confidence, taking into account the prevalence rate of the study population (Delhi/Rohtak). Samples were divided into two groups. Group 1, the EPTB group (n=105) was subdivided into 1) confirmed EPTB cases [n=25; smear positive (n=21) or culture positive (n=4)] and 2) clinically suspected EPTB cases (n=80) that were smear negative and culture negative yet suspected on the basis of imaging, clinical findings, histological/cytological observations, and response to anti-tubercular therapy (ATT). Group 2 was the control group (n=50), which comprised non-TB individuals.
Among 105 EPTB specimens, there were 64 pleural fluids, six ascitic fluids, 20 pus specimens from different origins, two lymph node aspirates, ten urine specimens, two pleural biopsies, and one synovial fluid specimen. Similarly, pleural fluids (n=23), pus (n=5), ascitic fluids (n=16), and urine (n=6) were collected from non-TB individuals with renal failure, trauma, or cancer as controls. All EPTB patients were in the age group of 15-60 years, and both sexes were represented. The inclusion criteria in the selection of TB pleuritis and TB lymphadenitis patients were 1) fever, cough, pleuritic pain, malaise, anorexia, and exudative pleural effusion with lymphocytic predominance on diagnostic tap and 2) untreated patients with peripheral (superficial) TB lymphadenitis of cervical or axillary regions. Similarly, the inclusion criteria for the selection of abdominal TB patients were fever and weight loss with one or more of the following: diarrhea persisting >1 month; ascites; abdominal lymphadenopathy or mesenteric masses on ultrasound; or hepatomegaly, splenomegaly, generalized pain, or tenderness persisting over 7 days. The exclusion criteria were 1) patients with a history of TB in the preceding 2 years, those already receiving ATT, those with other coexisting medical illnesses (e.g., HIV-positive individuals), pregnant women, and diabetes mellitus patients and 2) smear- and culture-negative suspected EPTB patients who did not respond to ATT even up to 8 weeks of treatment. All EPTB and non-TB specimens except urine were collected in the minor operation theater, following all standard aseptic surgical precautions of the participating institutes. All specimens were stored at 4℃ and processed within 24 h.
All clinical specimens including pleural biopsies (after homogenization) were digested and decontaminated using 1% N-acetyl-L-cysteine combined with 4% NaOH (NALC-NaOH). Briefly, an equal volume of NALC-NaOH solution was added to a clinical sample in a 15-mL centrifuge tube, mixed by inversion and incubated at room temperature for 20 minutes. Later, 0.067 M phosphate buffer (pH 6.8) was added, and the reaction mixture was subjected to centrifugation at 3000× g for 15 min. The supernatant was discarded, and the pellet was used for DNA extraction, smear microscopy, and the culture. The decontamination and centrifugation steps were performed in a biosafety hood. The mycobacterial genomic DNA was extracted according to the method of van Helden, et al.13 with few modifications using the cetyl-N,N,N-trimethyl ammonium bromide (CTAB)-phenol-chloroform method. Briefly, the pellet was suspended in 500 µL of Tris-EDTA buffer, heated at 100℃ for 10 minutes, and incubated with 20 µL of lysozyme (10 mg/mL) at 37℃ for 2 h. Then, 30 µL of 10% SDS and 10 µL of proteinase K (20 mg/mL) were added, briefly vortexed, and incubated at 37℃ for 1 hour, followed by incubation with 5 M NaCl (100 µL)+10% CTAB (80 µL) at 65℃ for 20 min. Later, an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) mixture was added, mixed by inversion and centrifuged at 14000× g for 10 minutes. This step was repeated twice for the removal of DNA contaminants. The supernatant was removed, followed by the addition of 3 M sodium acetate and isopropanol for DNA precipitation. The precipitate was washed with 70% ethanol and air dried. The DNA pellet was suspended in 100 µL of Tris-EDTA and stored at -20℃ until further analysis.
We designed primers for IS6110, mpb64, esat6, cfp10, 38kDa, 30kDa, devR, and hsp65 (targets of M. tuberculosis H37Rv) using Primer 3 software for PCR and validated the designs with a primer-designing software tool (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). All primers were obtained from Eurofins Genomic India Pvt. Ltd., Bengaluru. Table 1 shows the primer sequence as well as the annealing temperature used for different targets. For PCR amplification of individual gene targets, 1 µL of purified M. tuberculosis H37Rv DNA (1 ng/mL) was added to 25 µL of PCR master mix containing 2.5 µL 10× PCR buffer, 1.5 µL of 25 mM MgCl2, 0.5 µL of 200 µM (each) of the four deoxyribonucleoside triphosphates, 10 pM of each forward and reverse primer, PCR grade water, and 0.625 U of Taq DNA polymerase (Bangalore Genei Pvt. Ltd., Bengaluru, India). The DNA amplification was performed in a thermal cycler (T100™ BIO-RAD). After an initial denaturation at 95℃ for 3 min, each cycle was composed of three steps: denaturation at 95℃ for 30 s followed by annealing of respective primers at an appropriate temperature (Table 1) for 30 s, extension at 72℃ for 45 s for 30 cycles, and a final extension at 72℃ for 5 min. The PCR-amplified products were resolved by gel electrophoresis using 1.5% agarose in 1X TAE buffer containing ethidium bromide (0.5 µg/mL) at 80 volts for about 40 min and viewed under the Gel Documentation System (MiniLumi, DNR Bio-Imaging Systems Ltd., Jerusalem, Israel) to determine the presence of a specific size band for each gene target. The PCR amplification for DNA isolated from the clinical specimens was performed in a similar manner. All samples were run in duplicate. In each experiment, a positive control (M. tuberculosis DNA at 1 ng/mL) and two negative controls (no template DNA, only PCR grade water) were included.
M-PCR (mpb64+IS6110) amplification was performed with the same concentrations of master mix components as for monoplex PCR, with the exception of the different primer pair concentrations. DNA amplification was performed for 30 cycles as described above, although the annealing step was performed at 53℃ for 30 s. For the optimization of M-PCR, the combinations of different concentrations of two primer pairs were evaluated (Table 2) using 1 µL of purified M. tuberculosis DNA (1 ng/mL) to choose an appropriate ratio for mpb64:IS6110, which showed the clear amplicons of specific sizes for the two gene targets. PCR amplification for DNA isolated from the clinical specimens was performed in a similar manner and determined the presence or absence of a single band or two bands of specific size(s) under the Gel documentation system. Both positive and negative controls were run as explained above. The PCR/M-PCR results were compared using clinical diagnosis as the gold standard (comprising smear, culture, clinical findings, imaging, histological and cytological observations, response to ATT, and all together).
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated as detailed by Srivastava, et al.14
Prior to taking up this study in EPTB samples, validation of the PCR tests using different gene targets was carried out with few sputum samples (n=6) from PTB patients, which were found to be smear-positive and culture-positive for M. tuberculosis.
Fig. 1 shows the amplicons of different size(s) using different gene targets (IS6110, mpb64, esat6, cfp10, 38kDa, 30kDa, devR, and hsp65) with the purified M. tuberculosis DNA via PCR. After optimization of PCR with the purified DNA, we performed PCR using the clinical EPTB as well as non-TB samples. Representatives of positive clinical samples using different gene targets have been depicted (Fig. 1). The order of sensitivity with different targets in 105 EPTB cases (both confirmed and suspected cases) in descending order was mpb64>IS6110>hsp65>38kDa>30kDa>esat6>cfp10>devR (Table 3). However, the specificity of the PCR assay was high (88-100%) with all gene targets employed. The PPV, NPV, and accuracy observed with the individual gene targets have been summarized as percentages in Table 3.
Considering that mpb64 and IS6110 showed the highest positivity among all eight targets examined, we chose these two targets for M-PCR. We optimized the primer concentrations for mpb64+IS6110 (Table 2) to develop an M-PCR. Among the different combinations of primer concentrations, a combination of 0.2 µM of mpb64 and 0.4 µM of IS6110 showed sharp amplicons with the purified M. tuberculosis DNA (Fig. 2). Therefore, the same combination of primers was incorporated in the PCR master mixture to evaluate the clinical EPTB samples.
The M-PCR targeting mpb64+IS6110 showed better % sensitivity (90.5%) than monoplex PCR (Table 3 and 4) with individual mpb64 (79%) and IS6110 (75%) with a high specificity (100%) in 105 EPTB and 50 non-TB specimens (controls). Among the 25 confirmed EPTB cases, M-PCR revealed 24 positive cases, thus leading to 96% positivity. Out of 24 positive cases, 17 were positive with both IS6110 and mpb64, three were positive with IS6110 only, and four were positive with mpb64 only. Out of 80 clinically suspected EPTB cases, M-PCR showed 71 positive cases, thus leading to 88.75% positivity. Out of 71 positive cases, 50 were positive with both IS6110 and mpb64, nine were positive with IS6110 only, and 12 were positive with mpb64 only (Fig. 3). The M-PCR test demonstrated 100% PPV, 83.33% NPV, and 93.54% accuracy in 105 EPTB cases. The M-PCR results of individual EPTB specimens, including pleural fluids, pleural biopsies, pus, and urine, have been summarized in Table 5. Overall, the sensitivity with microscopy, culture, and M-PCR in 105 EPTB cases was 20%, 3.8%, and 90.47%, respectively, although high specificity (100%) was observed for all these tests in 50 non-TB specimens (controls).
The diagnosis of EPTB remains inconclusive in most cases, which can lead to serious consequences. Due to the inadequate sensitivities of conventional bacteriological methods, an unprecedented interest has been stimulated regarding the development of NAA tests such as PCR to facilitate an early diagnosis of EPTB. However, meta-analysis studies have revealed that heterogeneous sensitivities and specificities are observed with in-house PCR tests, while commercial PCR tests yield high specificities yet variable sensitivities.215 The major drawback of commercial tests is their high cost, which makes them unaffordable in most developing countries with a high TB burden.215 Furthermore, the true accuracy of PCR tests may actually be different than the reported one if using an imperfect gold or reference standard.612 Culture is the most widely used gold standard, though it is suboptimal for validating EPTB specimens with varying sensitivities, which can lead to inaccurate PCR results.216 Several researchers61617 used clinical diagnosis and composite reference standard (CRS; combination of smear, culture, histology and cytology, clinical findings, response to ATT, etc.) to validate PCR results when diagnosing EPTB specimens, and this study also used this validation method.
We chose mpb64 and IS6110 to develop an M-PCR, which revealed a high sensitivity both in confirmed (96%) and clinically suspected (88.75%) EPTB cases (Table 4). Our M-PCR test was able to detect 16 cases in which IS6110 was missing yet mpb64 was present. Similarly, there were 12 cases in which mpb64 was missing yet IS6110 was present (Table 4). No false positive results were observed with the 50 non-TB individuals, thus leading to 100% specificity. This was likely due to the utilization of highly specific M. tuberculosis-specific gene targets (mpb64 and IS6110) and also good laboratory practices, such as when handling the samples, as well as the inclusion of proper negative controls. Similar to our studies, high sensitivity and specificity has been documented for an M-PCR with mpb64+IS6110, designed for the diagnosis of TB lymphadenitis, osteoarticular TB, and gastrointestinal TB;101118 however, no report is available on TB pleuritis. We demonstrated high sensitivity (95.3%) and specificity (100%) for M-PCR using mpb64+IS6110 from pleural fluids (64 from TB patients and 23 from non-TB individuals) for the diagnosis of TB pleuritis (Table 5). Based on a meta-analysis of six studies (598 samples) on the Xpert assay, lower sensitivity (21.4%) has been reported for the diagnosis of TB pleuritis from pleural fluids, using CRS as the reference standard.17 Therefore, the World Health Organization (WHO) has not recommended the use of the Xpert assay for the diagnosis of TB pleuritis, though the same assay has been recommended for the diagnosis of TB lymphadenitis and TB meningitis.17 Interestingly, our M-PCR test was positive in six of ten urine samples collected from the suspected TB pleuritis patients (Table 5). Among those six positive cases, two were missed by IS6110 yet were detected by mpb64. The detection of M. tuberculosis in urine samples by PCR can be a useful method for the diagnosis of TB pleuritis and other clinical EPTB forms in which sample collection is difficult and requires aggressive techniques,19 though it needs further validation in a large number of specimens. The PCR/M-PCR assay is also limited in that it does not discriminate between alive and dead or degraded bacilli. However, this is likely a theoretical limitation, and its relevance must be judged in light of the overall picture of EPTB cases.
Genotype studies of M. tuberculosis strains in the Indian population using spoligotyping and IS6110-restriction fragment length polymorphism reveal the East African Indian family to be the major clade in the south India, whereas the Central Asian family (CAS) predominates in the northern part of India,2021 and the largest clade among the CAS belongs to the CAS1-Delhi lineage, which possesses variable IS6110 copy numbers. IS6110, which belongs to a family of IS of the IS3 category has been widely employed in PCR tests due to the presence of its multiple copies in the M. tuberculosis complex genome, which is believed to confer higher sensitivity.22 However, in recent years, several clinical investigations raised concerns over IS6110 specificity as well as sensitivity in the diagnosis of TB owing to false-positive (due to homology with other target DNA besides M. tuberculosis) or false-negative (due to a single copy or absence of copies of IS6110) results, particularly from Southeast Asian countries,26 thereby suggesting the use of other potential gene targets for monoplex or M-PCR assays. Our PCR results with individual IS6110 and mpb64 (Table 3) are similar to previous studies1016 yet differ from the other studies2324 reporting a sensitivity of <50%. Balne, et al.25 recently reported a very low sensitivity of 9% only with IS6110-based PCR in ocular TB specimens, whereas 70.2% and 40% sensitivities were observed with individual mpb64 and 38kDa, respectively. The reason for low sensitivity values with IS6110 in many studies could be due to south Indian isolates, 40% of which are shown to possess zero or low copy numbers of IS6110, while only 10-15% of North Indian isolates are shown to possess zero or low copy numbers of IS6110.222 Other reasons for low sensitivity with IS6110 or other targets could also relate to insufficient lysis of bacterial cells, loss of DNA during purification, or the presence of PCR inhibitors in the EPTB samples.
The fbpB gene (Rv1886c), encoding 30 kDa protein, was included in these assays, as this protein constitutes up to 41% of total mycobacterial proteins in log-phase culture supernatants. The mRNA coding for 30 kDa protein has been utilized as a target for reverse transcriptase-PCR26 in order to diagnose EPTB, and it can also detect viable mycobacteria; however, it is cumbersome to work with mRNA routinely. We performed PCR targeting the 30kDa gene (Table 3), which revealed a slightly lower sensitivity (70%) than earlier observations (87.5% sensitivity) made by Kidane, et al.27 when diagnosing TB lymphadenitis. The PCR results with the individual hsp65 and 38kDa gene targets (65.7-69.5% sensitivity) (Table 3) are almost similar to previous studies with EPTB specimens, such as pleural fluids, pus, and skin or abdomen biopsies.82628 When performed individually, our combined IS6110 PCR and devR PCR tests showed a sensitivity of 80.9% and a specificity of 88% in 105 EPTB specimens (Table 3), which is comparable to such combined PCR tests reported by Chakravorty, et al.6 in pleural fluids and lymph node biopsies using the universal sample processing method. However, M-PCR in a single tube has advantages, as it reduces errors of cross contamination as well as cost and increases the sensitivity of the test.
The simultaneous identification and differentiation of M. tuberculosis and M. avium complexes and the non-tuberculous mycobacteria directly from PTB and EPTB specimens have been documented for an M-PCR using genus-specific primers targeting hsp65, esat6/cfp10, and M. avium complex-specific primer pairs targeting 16S-23S rDNA internal transcribed spacer-1 sequences.2930 Similarly, Kim, et al.31 devised an M-PCR for the identification of M. tuberculosis complex to the species level in 37 strains and 178 clinical isolates targeting rpoB as well as RD1 and RD8 sequences. While performing PCR with the individual RD1 esat-6 and cfp10 gene targets for the diagnosis of EPTB specimens, lesser sensitivities (57-59%) (Table 3) were observed as compared to mpb64 or IS6110, likely due to the genetic variability of RD1 genes or low copies of ESAT-6 gene clusters in the genomes of certain M. tuberculosis isolates.3233 We recently detected RD1 and RD2 proteins (ESAT-6, MPB-64, etc.) from both PTB and EPTB specimens using a novel ultra-sensitive immuno-PCR assay (PCR amplified immunoassay) with a good diagnostic efficacy,34 though it needs to be better optimized for routine use.
Similar to our studies, Dubey, et al.35 used IS6110+mpb64 in designing an M-nested PCR for the detection of M. tuberculosis directly from the blood samples of PTB as well as EPTB patients, using a Blacklight Card Disc with high sensitivity (95.7%) and specificity (100%). However, Elnifro, et al.36 earlier suggested avoiding the use of nested primers for the M-PCR assay as this could lead to false positive results. Other researchers developed M-PCR using an IS6110+38kDa, IS6110+devR or IS6110+IS-like element B9, for the diagnosis of PTB and EPTB.373839 The combination of three gene targets (IS6110+dnaJ+hsp65 or IS6110+16S rRNA+devR) for designing an M-PCR has also been documented as a diagnosis tool for EPTB.940 However, we were unable to further raise the sensitivity of M-PCR using three gene targets, such as IS6110+mpb64+38kDa or IS6110+mpb64+hsp65 (data not shown), as our M-PCR exhibited substantially high sensitivity and specificity with two gene targets.
In conclusion, this study demonstrates the superiority of mpb64, followed by IS6110, among the several gene targets tested and further indicates the effectiveness of mpb64+IS6110 in the design of an efficient M-PCR test that facilitates an early diagnosis of smear-negative and culture-negative paucibacillary EPTB specimens, which are difficult to diagnose with the available standard methods. To the best of our knowledge, this is the first report to evaluate M-PCR with mpb64+IS6110 for the efficient diagnosis of TB pleuritis. Presently, we are validating these results in a large number of samples from varied clinical EPTB types in order for this simple and cost-effective test to be included in the diagnostic panel on a routine basis, which may prove to be a better alternative than the rpoB-based Xpert assay in resource-poor settings. This test might perform well in varied clinical EPTB types from various countries that harbor M. tuberculosis isolates with high copy numbers of IS6110 and also countries that harbor M. tuberculosis isolates with zero or low copy numbers of IS6110 (e.g., South Indian isolates), as most can be detected by mpb64.
Figures and Tables
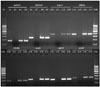 | Fig. 1PCR gel picture of several gene targets tested on the same clinical EPTB specimens. L1, L18, L19, and L36 represent 100 bp molecular marker; L2, L6, L10, L14, L20, L24, L28, and L32 were positive controls with the purified M. tuberculosis H37RvDNA; L3, L7, L11, L15, L21, L25, L29, and L33 were negative controls without template DNA; L4, L5, L8, L9, L12, L13, L16, L17, L22, L23, L26, L27, L30, L31, L34, and L35 represent clinical EPTB specimens. EPTB, extrapulmonary tuberculosis. |
 | Fig. 2M-PCR: amplification of 163 bp region of mpb64 gene and 258 bp region of IS6110 of M. tuberculosis H37RvDNA in the same tube with different ratios of primers. L1 represents 100 bp molecular marker; L2, L3, L4, and L5 represent mpb64 and IS6110 primer concentrations (µM) in ratios of 0.2:0.2, 0.2:0.4, 0.4:0.4, and 0.4:0.2 with M. tuberculosis H37Rv DNA; L6, negative control (no template DNA). M-PCR, multiplex-polymerase chain reaction. |
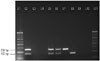 | Fig. 3M-PCR: amplification of 163 bp region of mpb64 gene and 258 bp region of IS6110 in the same tube; L1 and L11 represent 100 bp molecular marker; L2, positive control (M. tuberculosis H37Rv DNA); L3, negative control (no template DNA); L4, negative control (only PCR grade water); L5-8, representative positive clinical EPTB samples; L9-10, representative negative EPTB samples. M-PCR, multiplex-polymerase chain reaction; EPTB, extrapulmonary tuberculosis. |
Table 1
Primer Sequences Used for Different Gene Targets of M. tuberculosis H37Rv
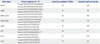
Table 2
Combination of Different Concentrations of Two Primer Pairs Used for Optimization of M-PCR

| Primer pairs | Concentration of primers (µM) | |||
|---|---|---|---|---|
| mpb64 | 0.2 | 0.2 | 0.4 | 0.4 |
| IS6110 | 0.2 | 0.4 | 0.4 | 0.2 |
Table 3
PCR Results Using Various Gene Targets

Table 4
Sensitivity and Specificity of M-PCR

Table 5
Sensitivity of M-PCR in Individual EPTB Specimens

ACKNOWLEDGEMENTS
The financial assistance provided by the University Grant Commission, New Delhi, the Department of Biotechnology (Builder-Programme), New Delhi and the Department of Science and Technology, New Delhi to carry out this work is duly acknowledged. We sincerely thank Prof. Jaya Tyagi, AIIMS, New Delhi for providing M. tuberculosis H37Rv DNA and Dr. Sunil Kumar, RBIPMT, Delhi for collecting EPTB samples.
References
1. World health organization. Global tuberculosis report. Geneva, Switzerland: World Health Organization;2014.
2. Mehta PK, Raj A, Singh N, Khuller GK. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol. 2012; 66:20–36.

3. RNTCP 2014 TB India. Revised national TB control program annual status report. New Delhi: Central TB Division;2014.
4. Mehta PK, Kalra M, Khuller GK, Behera D, Verma I. Development of an ultrasensitive polymerase chain reaction-amplified immunoassay based on Mycobacterial RD antigens: implications for the serodiagnosis of tuberculosis. Diagn Microbiol Infect Dis. 2012; 72:166–174.

5. Makeshkumar V, Madhavan R, Narayanan S. Polymerase chain reaction targeting insertion sequence for the diagnosis of extrapulmonary tuberculosis. Indian J Med Res. 2014; 139:161–166.
6. Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005; 43:4357–4362.

7. Haldar S, Bose M, Chakrabarti P, Daginawala HF, Harinath BC, Kashyap RS, et al. Improved laboratory diagnosis of tuberculosis--the Indian experience. Tuberculosis (Edinb). 2011; 91:414–426.
8. Negi SS, Anand R, Basir SF, Pasha ST, Gupta S, Khare S, et al. Protein antigen b (Pab) based PCR test in diagnosis of pulmonary and extra-pulmonary tuberculosis. Indian J Med Res. 2006; 124:81–88.
9. Bandyopadhyay D, Gupta S, Banerjee S, Gupta S, Ray D, Bhattacharya S, et al. Adenosine deaminase estimation and multiplex polymerase chain reaction in diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008; 12:1203–1208.
10. Sharma K, Sinha SK, Sharma A, Nada R, Prasad KK, Goyal K, et al. Multiplex PCR for rapid diagnosis of gastrointestinal tuberculosis. J Glob Infect Dis. 2013; 5:49–53.

11. Sharma K, Gupta N, Sharma A, Singh G, Gupta PK, Rajwanshi A, et al. Multiplex polymerase chain reaction using insertion sequence 6110 (IS6110) and Mycobacterial protein fraction from BCG of Rm 0.64 in electrophoresis target genes for diagnosis of tuberculous lymphadenitis. Indian J Med Microbiol. 2013; 31:24–28.

12. Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012; 40:442–447.

13. van Helden PD, Victor TC, Warren RM, van Helden EG. Isolation of DNA from Mycobacterium tubercolosis. Methods Mol Med. 2001; 54:19–30.
14. Srivastava R, Kumar D, Waskar MN, Sharma M, Katoch VM, Srivastava BS. Identification of a repetitive sequence belonging to a PPE gene of Mycobacterium tuberculosis and its use in diagnosis of tuberculosis. J Med Microbiol. 2006; 55(Pt 8):1071–1077.

15. Pai M, Flores LL, Hubbard A, Riley LW, Colford JM Jr. Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis. BMC Infect Dis. 2004; 4:6.

16. Vadwai V, Shetty A, Rodrigues C. Using likelihood ratios to estimate diagnostic accuracy of a novel multiplex nested PCR in extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2012; 16:240–247.

17. Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014; 44:435–446.

18. Sharma K, Sharma A, Sharma SK, Sen RK, Dhillon MS, Sharma M. Does multiplex polymerase chain reaction increase the diagnostic percentage in osteoarticular tuberculosis? A prospective evaluation of 80 cases. Int Orthop. 2012; 36:255–259.

19. Rebollo MJ, San Juan Garrido R, Folgueira D, Palenque E, Díaz-Pedroche C, Lumbreras C, et al. Blood and urine samples as useful sources for the direct detection of tuberculosis by polymerase chain reaction. Diagn Microbiol Infect Dis. 2006; 56:141–146.

20. Varma-Basil M, Kumar S, Arora J, Angrup A, Zozio T, Banavaliker JN, et al. Comparison of spoligotyping, Mycobacterial interspersed repetitive units typing and IS6110-RFLP in a study of genotypic diversity of Mycobacterium tuberculosis in Delhi, North India. Mem Inst Oswaldo Cruz. 2011; 106:524–535.

21. Gupta A, Kulkarni S, Rastogi N, Anupurba S. A study of Mycobacterium tuberculosis genotypic diversity & drug resistance mutations in Varanasi, North India. Indian J Med Res. 2014; 139:892–902.
22. Sankar S, Kuppanan S, Balakrishnan B, Nandagopal B. Analysis of sequence diversity among IS6110 sequence of Mycobacterium tuberculosis: possible implications for PCR based detection. Bioinformation. 2011; 6:283–285.

23. Jin XJ, Kim JM, Kim HK, Kim L, Choi SJ, Park IS, et al. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn's disease. World J Gastroenterol. 2010; 16:2496–2503.

24. Rana T, Singh UB, Kulshrestha V, Kaushik A, Porwal C, Agarwal N, et al. Utility of reverse transcriptase PCR and DNA-PCR in the diagnosis of female genital tuberculosis. J Med Microbiol. 2011; 60(Pt 4):486–491.

25. Balne PK, Modi RR, Choudhury N, Mohan N, Barik MR, Padhi TR, et al. Factors influencing polymerase chain reaction outcomes in patients with clinically suspected ocular tuberculosis. J Ophthalmic Inflamm Infect. 2014; 4:10.

26. Negi SS, Anand R, Pasha ST, Gupta S, Basir SF, Khare S, et al. Diagnostic potential of IS6110, 38kDa, 65kDa and 85B sequence-based polymerase chain reaction in the diagnosis of Mycobacterium tuberculosis in clinical samples. Indian J Med Microbiol. 2007; 25:43–49.

27. Kidane D, Olobo JO, Habte A, Negesse Y, Aseffa A, Abate G, et al. Identification of the causative organism of tuberculous lymphadenitis in ethiopia by PCR. J Clin Microbiol. 2002; 40:4230–4234.

28. Kulkarni S, Vyas S, Supe A, Kadival G. Use of polymerase chain reaction in the diagnosis of abdominal tuberculosis. J Gastroenterol Hepatol. 2006; 21:819–823.

29. Gopinath K, Singh S. Multiplex PCR assay for simultaneous detection and differentiation of Mycobacterium tuberculosis, Mycobacterium avium complexes and other Mycobacterial species directly from clinical specimens. J Appl Microbiol. 2009; 107:425–435.

30. Chia JH, Wu TL, Su LH, Kuo AJ, Lai HC. Direct identification of Mycobacteria from smear-positive sputum samples using an improved multiplex polymerase chain reaction assay. Diagn Microbiol Infect Dis. 2012; 72:340–349.

31. Kim Y, Choi Y, Jeon BY, Jin H, Cho SN, Lee H. A simple and efficient multiplex PCR assay for the identification of Mycobacterium genus and Mycobacterium tuberculosis complex to the species level. Yonsei Med J. 2013; 54:1220–1226.

32. Gey Van Pittius NC, Gamieldien J, Hide W, Brown GD, Siezen RJ, Beyers AD. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C gram-positive bacteria. Genome Biol. 2001; 2:RESEARCH0044.
33. Uplekar S, Heym B, Friocourt V, Rougemont J, Cole ST. Comparative genomics of esx genes from clinical isolates of Mycobacterium tuberculosis provides evidence for gene conversion and epitope variation. Infect Immun. 2011; 79:4042–4049.

34. Mehta PK, Raj A, Singh NP, Khuller GK. Detection of potential microbial antigens by immuno-PCR (PCR-amplified immunoassay). J Med Microbiol. 2014; 63(Pt 5):627–641.

35. Dubey A, Gwal R, Agrawal S. Mycobacterium tuberculosis detection in blood using multiplex nested polymerase chain reaction. Int J Tuberc Lung Dis. 2013; 17:1341–1345.

36. Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000; 13:559–570.

37. Kulkarni S, Singh P, Memon A, Nataraj G, Kanade S, Kelkar R, et al. An in-house multiplex PCR test for the detection of Mycobacterium tuberculosis, its validation & comparison with a single target TB-PCR kit. Indian J Med Res. 2012; 135:788–794.
38. Sharma SK, Sethi S, Sharma M, Meharwal SK, Katoch VM, Jindal SK, et al. Development and evaluation of a multiplex polymerase chain reaction for the detection of Mycobacterium tuberculosis from pulmonary specimens. Scand J Infect Dis. 2012; 44:739–744.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download