Abstract
Purpose
To examine the usefulness of various receptor tyrosine kinase expressions as prognostic markers and therapeutic targets in muscle invasive urothelial cancer (UC) patients.
Materials and Methods
We retrospectively analyzed the data of 98 patients with muscle invasive UC who underwent radical cystectomy between 2005 and 2010 in Yonsei Cancer Center. Using formalin fixed paraffin embedded tissues of primary tumors, immunohistochemical staining was done for human epidermal growth factor receptor 2 (HER2), fibroblast growth factor receptor 1 (FGFR1), and fibroblast growth factor receptor 3 (FGFR3).
Results
There were 41 (41.8%), 44 (44.9%), and 14 (14.2%) patients who have over-expressed HER2, FGFR1, and FGFR3, respectively. In univariate analysis, significantly shorter median time to recurrence (TTR) (12.9 months vs. 49.0 months; p=0.008) and overall survival (OS) (22.3 months vs. 52.7 months; p=0.006) was found in patients with FGFR1 overexpression. By contrast, there was no difference in TTR or OS according to the HER2 and FGFR3 expression status. FGFR1 remained as a significant prognostic factor for OS with hazard ratio of 2.23 (95% confidence interval: 1.27–3.90, p=0.006) in multivariate analysis.
Urothelial carcinoma (UC) is the fourth most common malignancy in men and the ninth most common in women, accounting for estimated new cases of 330400 and estimated death of 123100 patients worldwide in 2012.1 There are two distinct subtypes in UC: superficial disease and muscle invasive disease.23 Almost 80% of patients with UC are initially diagnosed with superficial disease which is potentially curable by transurethral resection. Nevertheless, the majority of them experience one or more recurrences, and 25% will eventually develop muscle invasive disease. Although the standard treatment of muscle invasive disease is radical cystectomy, the majority of treatment failure is systemic relapse with distant metastases.4 Since gemcitabine plus cisplatin demonstrated equivalent efficacy with superior tolerability compared to methotrexate, vinblastine, doxorubicin, and cisplatin in randomized phase III trial, it has become the standard treatment of advanced or metastatic UC.5 However, there is no standard therapeutic option after failure of cisplatin-based first line chemotherapy, which rendered the overall survival (OS) of patients with advanced UC less than 2 years. Thus, there is clearly unmet need to identify molecular markers and to develop more effective therapeutic strategy for UC.
Human epidermal growth factor-2 receptors (HER2) play crucial role in signal transduction pathways regarding cell proliferation, survival, angiogenesis, and metastasis. Since trastuzumab, a recombinant humanized monoclonal antibody targeted to the extracellular domain of HER2 have dramatically changed the treatment of HER2 amplified breast cancer and gastric cancer,678 there is emerging interest to apply this therapeutic strategy to other malignancies. Although HER2 amplification and overexpression were found in UCs, there exists higher variability ranging from 23% to 80% for over-expression and from 0% to 32% for amplification.910 In addition, its role as a prognostic marker in muscle invasive UC still remains controversial.11121314
Fibroblast growth factor receptors (FGFRs) play a key role in the regulation of proliferation, differentiation, and apoptosis.15 Recently, it has become clear that there exists FGF signaling alteration in a substantial proportion of bladder tumors.16 Mutation of FGFR3, the most common genetic alteration in superficial UC, results in constitutive activation of the receptor,17 and it is strongly associated with low tumor grade and stage.18 While increased expression of FGFR3 was found in the majority (about 85%) of FGFR3-mutant superficial tumors, it may also be achieved via overexpression of the wild-type receptor.18 Muscle invasive bladder tumors have been found to overexpress wild type FGFR3 protein, as well.18 These results suggest a potential role of mutant FGFR3 predominantly in superficial UC and overexpression of wild-type FGFR3 in invasive UC. Preclinical studies using small molecule inhibitor against FGFR showed the possibility of FGFR3 as a useful therapeutic target in UC.19 Although relatively little is known about the role of other FGFRs in UC, FGFR1 expression is known to be increased in the majority of bladder cancer cell lines regardless of tumor stage and grade,20 and increased FGFR1 expression induces increased proliferation and cell survival.20 However, there has been no data regarding the prognostic role of FGFR1 and FGFR3 overexpression in patients with muscle invasive UC after radical cystectomy.
Hence, the aim of this study is to examine the usefulness of HER2, FGFR1, and FGFR3 expression as prognostic markers and therapeutic targets in muscle invasive UC patients. In addition, we investigated the correlations between these receptor tyrosine kinases (RTKs) and various clinico-pathological factors.
We retrospectively analyzed the data of 98 patients who underwent radical cystectomy for muscle invasive UC of the urinary bladder from 2005 to 2010 at a single institute, Yonsei Cancer Center. We included patients whose archival tissue samples from cystectomy specimen were available, and tumor histology should be confirmed as UC. Patients were excluded if they receive neoadjuvant chemotherapy before they underwent radical cystectomy. All patients underwent radical cystectomy and bilateral pelvic lymphadenectomy. Surgical procedures consisted of an en-bloc radical cystectomy with para-aortic lymph node dissection (PLND) and urinary diversion. PLND included the internal and external iliac and obturator lymph nodes. Tumors were restaged according to the American Joint Commmitee on Cancer/International Union against Cancer staging system 7th edition.21 World Health Organization reference center system was used for tumor grading.
This study received the approval of Institutional Review Board and was conducted in accordance with the Helsinki Declaration of 1975.
For immunohistochemistry (IHC) analysis, formalin-fixed paraffin-embedded tissue containing the representatives of each tumor were prepared and sectioned as slides with 5-um thickness. Slides were stained with the monoclonal antibodies: FGFR1 (Rabbit Anti-Human FGFR-1 polyclonal antibody, SPRING, Pleasanton, CA, USA), FGFR3 [FGFR-3 (B-9), Santa Cruz, Dallas, TX, USA], and HER2 (polyclonal rabbit anti-human c-erbB-2 oncoprotein, DAKO, Glostrup, Denmark) via standard IHC as previously described.18
The level of HER2 protein expression was assessed by the intensity and percentage of staining and scored on a scale of 0 to 3+.9 The evaluation was performed only on the invasive component of the tumor. A score of 1+ was defined as barely perceptible membrane staining in more than 10% of cells, a score 2+ was defined as weak-to-moderate complete membrane staining present in more than 10% of tumor cells, and a score 3+ was defined as strong complete membrane staining in more than 30% of tumor cells. A cytoplasmic staining was considered nonspecific. Tumors presenting 2+ or 3+ HER2 expression were considered to have HER2 overexpression.
A semi-quantitiative scoring system was adopted: 0, all tumor cells negative; 1, faint but detectable positivity in some or all cells; 2, weak but extensive positivity; 3, strong positivity.18 As there is yet no established method for the assessment for FGFR1 or FGFR3 positivity, we defined FGFR1 and FGFR3 positive if they have cytoplasmic immune-reactivity regardless of the staining intensity (Fig. 1). Immunostaining was assessed by two independent pathologists who were blinded to clinical outcomes.
OS was calculated from the date of cystectomy to death of any cause. Time to recurrence (TTR) was defined as the duration from cystectomy to the date of first documented recurrence. Patients who were still alive at the cut off day were censored at the date of last contact.
The association between clinico-pathological parameters was evaluated using Student's t-test (for numerical variables) or Pearson's chi-square test (for categorized variables). Karplan-Meier curves were plotted for TTR and OS, and the difference of survival time was analyzed using log-rank test. Multivariate survival analysis was performed with Cox's proportional regression hazard model. p values <0.05 were used as significant level. All analyses were performed with SPSS program, version 18.0 (SPSS Inc., Chicago, IL, USA).
The baseline characteristics of the patients are described in Table 1. The median age was 69.5 years (range, 41–88 years), with 82 male and 16 female patients. Sixty four patients (65.3%) had muscle invasive disease as initial manifestation, whereas 34 patients (34.7%) were initially diagnosed with non-invasive disease, treated by transurethral resection of tumor one or more times, but ultimately developed muscle invasive disease.
Regarding pathologic stage after cystectomy, 23 patients (23.5%) were diagnosed with tumor confined to muscularis propria layer (T2) and tumor invasion beyond that (T3, T4) was observed in 75 patients (76.5%). Node involvement was found in 36 patients (36.7%). Overall, 58 patients (59.2%) were diagnosed with stage II or III disease, and 40 patients (40.8%) had stage IV disease. All the tumors were assessed as high grade tumor. After cystectomy, adjuvant chemotherapy was given to 47 patients (48.0%).
There were 41 (41.8%), 44 (44.9%), and 14 (14.2%) patients who had over-expressed HER2, FGFR1, and FGFR3, respectively (Fig. 2, Table 1). While 28 patients (28.6%) had none of them, 70 patients (71.4%) had at least one receptor expression among the three receptors. There were 19 patients who had over-expression of both HER2 and FGFR1, 5 patients in HER2 and FGFR3, and 8 patients in FGFR1 and FGFR3. Over-expression of all three receptors was found only in three patients.
With a median follow-up duration of 34.3 months (range 1–117 months), there has been 54 (55.1%) recurrences and 67 (67.7%) deaths. Median TTR and median OS of whole population was 18.2 months [95% confidence interval (CI): 12.8–23.5] and 37.3 months (95% CI: 21.7–52.9), respectively.
We performed univariate analysis of TTR and OS according to receptor status and other clinical parameters (Table 2). Significantly shorter median TTR was seen in female patients (compared to male, 7.5 months vs. 19.7 months; p=0.029), hemoglobin (Hb) ≤12.0 g/dL (compared to Hb >12.0 g/dL, 15.3 months vs. 30.9 months; p=0.021), lymph node positive (LN+) disease (compared to LN-disease, 15.1 months vs. 60.8 months; p=0.002), lympho-vascular invasion (compared to LVI-, 13.5 months vs. 38.4 months; p=0.002), and non-invasive disease as an initial manifestation (compared to initial invasive disease, 15.1 months vs. 23.3 months; p=0.027).
Regarding the receptor status, patients with FGFR1 overexpression had worse TTR than those without FGFR1 expression (12.9 months vs. 49.0 months; p=0.008) (Fig. 3A). By contrast, there was no difference in TTR according to HER2 and FGFR3 receptor status as well as age, previous adjuvant chemotherapy.
Similar results were found in univariate analysis for OS; female patients (compared to male, 11.2 months vs. 40.5 months; p=0.021), non-invasive disease as initial manifestation compared to invasive disease (19.1 months vs. 48.7 months; p=0.026), Hb ≤12.0 g/dL (compared to >12 g/dL, 26.5 months vs. 48.9 months; p=0.029), dissected lymph node number ≤12 (compared to >12, 19.3 months vs. 52.7 months; p=0.005) and FGFR1 overexpression (22.3 months vs. 52.7 months; p=0.006) showed significantly worse survival (Fig. 3B).
In multivariate analysis (Table 3), FGFR1 and non-invasive disease as initial manifestation remained as significant prognostic factors for TTR with hazard ratio of 2.04 (95% CI: 1.27–3.90, p=0.018) and 2.10 (95% CI: 1.28–4.17, p=0.008), respectively. Also, they were significant prognostic factor for OS with hazard ratio of 2.23 (95% CI: 1.27–3.90, p=0.006) and 1.80 (95% CI: 1.28–4.17, p=0.038), respectively.
We also examined whether there are any interactions of each receptor status with survival. In patients without FGFR1 expression, HER2 overexpression had a tendency for shorter TTR (16.7 months vs. not reached; p=0.085) and OS (42.7 months vs. 76.3 months; p=0.172). Interestingly, among patients with FGFR1 expression, HER2 overexpression showed longer TTR (15.3 months vs. 12.9 months; p=0.208) and OS (28.6 months vs. 20.2 months; p=0.142) without statistical significance.
Table 4 shows significant difference in the frequency of FGFR1 expression between gender; FGFR1 overexpression was found in 75% (12 out of 16) of female patients, whereas in only 39% (32 out of 50) of male patients (p=0.008). Also, FGFR1 overexpression was found more frequently in node positive patients than in node negative patients (61.1% vs. 35.5%; p=0.014). Consequently, it is more frequently found in stage IV than stage II or III patients (57.9% vs. 36.7%; p=0.040). Otherwise, there was no significant association in other variables according to receptor status.
We investigated the prognostic role of three different RTKs in the survival of the patients who underwent radical cystectomy for muscle invasive UC, and found that FGFR1 was a strong prognostic factor, whereas HER2 and FGFR3 did not show any prognostic impact. Although the association of activating mutations and over-expression of FGFR3 with a lower risk of progression and better survival in superficial UC have been well known,16 we herein first demonstrated the prognostic importance of FGFR1 in muscle invasive UC.
Recently, a lot of interest have been focused on FGFR pathway as a therapeutic target in UC because of earlier preclinical studies19 and the development of new drugs targeting FGFR pathway. Several clinical trials to investigate the efficacy of FGFR inhibitors in UC as single drug (NCT00790426, NCT01732107) or in combination with cytotoxic agents (NCT01496534) are underway. Currently, however, it is not known whether FGFRs have prognostic impact in muscle invasive UC. Our present study, first demonstrated that overexpression of FGFR1, but not FGFR3 or HER2, is a worse prognostic factor in muscle invasive UC: the prognostic impact of FGFR1 was sustained even after adjustment of other prognostic factors such as pathologic stage, lympho-vascular invasion, number of dissected lymph nodes, or Hb level before cystectomy.
One of important issues to be addressed is how to identify target population who can benefit the most from FGFR targeting drugs. Urothelial cancer cell lines with overexpression of FGFR1 or 3 respond to FGFR inhibitors more efficiently than cell lines with mutation of those receptors.19 To date, fluorescent in situ hybridization (FISH) or silver in situ hybridization has generally been used as detection methods for FGFR amplification in gastric cancer, breast cancer, and non-small cell lung cancer.222324 In the present study, however, we used IHC, one of the most available and inexpensive tools. The previous research showed low proportion of tumors showing FGFR1 amplification in UC which could not explain the high frequency of increased expression. This discrepancy suggests that FGFR1 overexpression might result from post-transcriptional regulation such as altered splicing or increased transcript stability rather than dependent only on gene amplification.25 Thus, we hypothesized that IHC which detects the final protein product could be a suitable method for the detection of FGFR1 overexpression in UC. Since we showed the prognostic role of FGFR1 overexpression assessed by IHC, IHC would be considered relevant technique to detect FGFR1 overexpression, at least in muscle invasive UC. However, further researches on true incidence of FGFR1 amplification (assessed by FISH) and the concordance rate between IHC and FISH are warranted to identify proper biomarker. Also, it needs to be validated in clinical trials targeting UC patients.
It was of an interest to observe significant difference in the frequency of FGFR1 over-expression tumors according to gender (female 75% vs. male 39%). In addition, female patients had significantly worse TTR and OS than male patients. The fact that it lost its prognostic impact after adjustment by other variables, including FGFR1, suggests that the worse survival outcome in female patients might be due to higher proportion of FGFR1 overexpression. Although this finding should be validated in a large, different cohort, it might help us to identify a subset of patient population who are more likely to have FGFR1 overexpression and benefit from therapeutic approach targeted to this molecular aberration, as female predominance of epidermal growth factor receptor mutation in non-small cell lung cancer did.
HER2 overexpression was observed in 41 out of 98 patients (41.8%), which was in the range reported in previous works. The high variability of HER2 overexpression incidence and its prognostic role can be explained by small numbers of patients in each study and also by heterogeneity of laboratory tests used. To overcome such limitations, the assessment of HER2 status needs to be standardized in terms of antibodies and interpretation of the results. In this study, HER2 overexpression did not show any difference on the survival of patients with muscle invasive UC after radical cystectomy. Interestingly, our result suggested that patients with HER2 overexpression tended to have better or worse survival outcome with or without FGFR1 receptor expression, respectively. Although the survival difference was not statistically significant, the discrepancies on the prognostic value of HER2 overexpression in UC, reported in previous articles, might be due to heterogeneity of FGFR1 status which was not checked in the previous works. If this is the case, UC patients could be stratified to four different categories according to HER2 and FGFR receptor status. However, one should be cautious to accept this hypothesis until it is validated in subsequent trials.
We hypothesized that there might be survival differences between muscle invasive tumors as initial presentation and initial superficial tumors in which muscle invasion developed later on. Notably, significant difference in both TTR and OS was seen between two groups. This finding might result from distinct pathophysiology at molecular level between two groups, or it might have simply been derived from treatment related effect. Although this results suggest that initial manifestation should be considered as important prognostic factor in patients who underwent radical cystectomy for muscle invasive UC, one should be cautious because we defined non-invasive disease based on pathologic finding after transurethral resection of bladder, a procedure with an inherent limitation for true pathologic staging. Further analysis of the treatment course may explain such disparity of outcome, however, we did not perform such analysis because substantial proportion of patients were diagnosed and treated with superficial disease before being transferred to the study hospital, or they were diagnosed a long time before cystectomy that we could obtain the medical record.
This study has several limitations, largely due to its retrospective nature. First, this study was conducted at a single center with a small number of patients. Thus, it is hard to generalize the results to an entire patient cohort. Second, because this study was based on retrospective chart review, there might be significant selection bias. Third, even though treatment was administered largely based on guidelines, there were some differences in treatment protocols and follow up strategies, such as interval at which follow-up CT scan was performed.
In conclusion, we showed that overexpression of FGFR1, but not FGFR3, assessed by IHC is strongly associated with disease recurrence and worse OS in muscle invasive UC patients who received radical cystectomy. Since it is found in a high proportion of invasive tumors and there are commercially available drugs targeted to this pathway, it should be considered as an important therapeutic target in future treatment strategy.
Figures and Tables
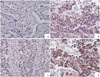 | Fig. 1Immunohistochemistry staining (×200) of urothelial carcinoma showing different grades of FGFR1 expression. (A) Negative. (B) Moderate signaling in 30% of cells. (C) Weak signaling in 70% of cells. (D) Moderate signaling in 70% of cells. FGFR1, fibroblast growth factor receptor 1. |
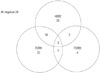 | Fig. 2Distribution of patients according to the HER2 and FGFR expression status. HER2, human epidermal growth factor receptor 2; FGFR, fibroblast growth factor receptor. |
 | Fig. 3Survival curves according to FGFR1 expression level. (A) Progression free survival. (B) Overall survival. FGFR1, fibroblast growth factor receptor 1. |
Table 1
Baseline Characteristics of Patients

Table 2
Univariate Analysis for TTR and OS

Table 3
Multivariate Analysis for TTR and OS

Table 4
Patient Characteristics According to Receptor Tyrosine Kinase

ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI13C2096).
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Knowles MA. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis. 2006; 27:361–373.

4. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001; 19:666–675.

5. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077.

6. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–792.

7. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005; 353:1673–1684.

8. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697.

9. Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010; 21:815–819.

10. Marín AP, Arranz EE, Sánchez AR, Auñón PZ, Barón MG. Role of anti-Her-2 therapy in bladder carcinoma. J Cancer Res Clin Oncol. 2010; 136:1915–1920.

11. Mellon JK, Lunec J, Wright C, Horne CH, Kelly P, Neal DE. CerbB-2 in bladder cancer: molecular biology, correlation with epidermal growth factor receptors and prognostic value. J Urol. 1996; 155:321–326.

12. Lönn U, Lönn S, Friberg S, Nilsson B, Silfverswärd C, Stenkvist B. Prognostic value of amplification of c-erb-B2 in bladder carcinoma. Clin Cancer Res. 1995; 1:1189–1194.
13. Vollmer RT, Humphrey PA, Swanson PE, Wick MR, Hudson ML. Invasion of the bladder by transitional cell carcinoma: its relation to histologic grade and expression of p53, MIB-1, c-erb B-2, epidermal growth factor receptor, and bcl-2. Cancer. 1998; 82:715–723.

14. Kolla SB, Seth A, Singh MK, Gupta NP, Hemal AK, Dogra PN, et al. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int Urol Nephrol. 2008; 40:321–327.

15. L'Hôte CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005; 304:417–431.
16. di Martino E, Tomlinson DC, Knowles MA. A decade of FGF receptor research in bladder cancer: past, present, and future challenges. Adv Urol. 2012; 2012:429213.

17. Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001; 2:REVIEWS3005.
18. Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007; 213:91–98.

19. Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011; 104:75–82.

20. Tomlinson DC, Lamont FR, Shnyder SD, Knowles MA. Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer. Cancer Res. 2009; 69:4613–4620.

21. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.
22. Kim HR, Kim DJ, Kang DR, Lee JG, Lim SM, Lee CY, et al. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J Clin Oncol. 2013; 31:731–737.

23. Jung EJ, Jung EJ, Min SY, Kim MA, Kim WH. Fibroblast growth factor receptor 2 gene amplification status and its clinicopathologic significance in gastric carcinoma. Hum Pathol. 2012; 43:1559–1566.

24. Jang M, Kim E, Choi Y, Lee H, Kim Y, Kim J, et al. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012; 14:R115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download