Abstract
Purpose
The aim of this study was to evaluate the efficacy of re-irradiation in patients with recurrent gliomas and to identify subgroups for whom re-irradiation for recurrent gliomas is most beneficial.
Materials and Methods
We retrospectively reviewed 36 patients with recurrent or progressive gliomas who received re-irradiation between January 1996 and December 2011. Re-irradiation was offered to recurrent glioma patients with good performance or at least 6 months had passed after initial radiotherapy (RT), with few exceptions.
Results
Median doses of re-irradiation and initial RT were 45.0 Gy and 59.4 Gy, respectively. The median time interval between initial RT and re-irradiation was 30.5 months. Median overall survival (OS) and the 12-month OS rate were 11 months and 41.7%, respectively. In univariate analysis, Karnofsky performance status (KPS) ≥70 (p<0.001), re-irradiation dose ≥45 Gy (p=0.040), and longer time interval between initial RT and re-irradiation (p=0.040) were associated with improved OS. In multivariate analysis, KPS (p=0.030) and length of time interval between initial RT and re-irradiation (p=0.048) were important predictors of OS. A radiographically suspected mixture of radiation necrosis and progression after re-irradiation was seen in 5 patients.
Gliomas have diverse prognoses that differ primarily depending on histologic subtype and grade. The mainstay of treatment is surgical resection followed by radiotherapy (RT) and/or chemotherapy; however, even after aggressive multimodal treatment including RT, most gliomas eventually recur.12345 Surgery, if applicable, is most efficacious salvage treatment method, but is limited to selected cases.6 Various drugs have been used for gliomas without effect.7891011 Re-irradiation has been tried in spite of the risk of radiation toxicity;121314151617181920 however, sufficient information on re-irradiation is not available because of a lack of randomized phase III trials and prospective multi-institutional studies. Therefore, many clinical questions about the optimal use of re-irradiation for gliomas are still unanswered.
At our institution, re-irradiation has been used for patients with recurrent glioma. We analyzed treatment outcomes and prognostic factors of survival for patients with recurrent gliomas treated with re-irradiation. We identified subgroups that benefit the most from re-irradiation for recurrent glioma.
Between January 1996 and December 2011, 36 patients (age ≥18 years) received re-irradiation for recurrent glioma at the Department of Radiation Oncology in our institution. Data were collected through retrospective review of medical records. We used the World Health Organization (WHO) grading system of primary brain tumors. As re-irradiation for gliomas was not standard treatment, there were no strict indications in selecting patients for re-irradiation. The patients were evaluated by the multidisciplinary neuro-oncology team, and re-irradiation was offered to patients if they had shown good performance or if at least 6 months had passed after initial RT, with few exceptions granted only at the discretion of the radiation oncologist. As recurrent tumors after irradiation are difficult to control by re-irradiation alone and often cannot be differentiated from radiation necrosis (RN), we attempted to apply maximal surgical resection with pathological confirmation of recurrent tumors before re-irradiation. Surgical tissues for recurrent gliomas were evaluated retrospectively to identify O6-methylguanine-DNA methyltransferase (MGMT) gene promoter methylation status. Genomic DNA was isolated from available paraffin-embedded samples from recurrent glioma patients.
Patients underwent simulation in the supine position and were immobilized with an aquaplastic mask device. Patients underwent treatment-planning computed tomography (CT) with 2–3 mm slices. In general, gross tumor volume (GTV) was defined as the contrast enhanced lesion or operation cavity plus 5 mm margin by magnetic resonance imaging (MRI) which was combined with the treatment-planning CT. Clinical target volume (CTV) was typically defined as GTV plus a 15–20 mm margin, and all instances of edema were not included in CTV. Planning target volume (PTV) was defined as CTV plus a 3-mm margin. RT was delivered using a Co-60 teletherapy unit, 4–6 MV linear accelerator that applied three to five noncoplanar isocentric fields that were irregularly shaped using a multileaf collimator with a leaf thickness of 5 mm at the isocenter or a helical tomotherapy Hi-Art System, version 2.0 (TomoTherapy Inc., Madison, WI, USA). In general, daily fraction doses of 1.8–2.0 Gy were prescribed for PTV. Total doses of re-irradiation were prescribed at the discretion of the radiation oncologist, who considered the cumulative dose between initial radiation and re-irradiation, re-irradiation target volume, and patient performance. The treatment dose was prescribed to the isocenter. To compensate for varying dose-fractionation schedules, radiation doses were expressed as biological equivalent dose (BED) values according to the linear-quadratic equation, where BED=D [1+d/(α/β)]. In this formulation, α/β is a radiobiological constant that is considered to be 2 Gy for normal brain tissue.
Baseline evaluations at recurrence included gadolinium-enhanced brain MRI, physical examination, and neurologic examination before re-irradiation. Treatment outcome was evaluated by brain MRI according to the Macdonald criteria21 and by neurologic status after re-irradiation. Progression-free survival(PFS) was defined as length of time from date of first re-irradiation to any recurrence or death; overall survival (OS) was defined as length of time from date of first re-irradiation to any cause of death. Treatment-related toxicities were evaluated at each follow-up visit. We defined acute and late toxicities as events occurring within 3 months and after 3 months from the start of re-irradiation, respectively. We evaluated late toxicity, including both RN and neurological deterioration. The assessment of other late toxicity was unavailable due to retrospective nature of the related limited medical records. All toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 4.0) and toxicities rated higher than grade 3 were considered severe.
The median age of patients was 42.5 years (range, 20–63 years) at the time of re-irradiation, and the study included 19 men and 17 women. The median Karnofsky performance status (KPS) at recurrence was 60. Of the patients, 17 (47.2%) had a KPS greater than 70, and six (16.7%) had a KPS below 40. Histologic subtypes at recurrence were verified through surgical resection or biopsy for 28 patients (77.8%): 18 glioblastomas (50%), six anaplastic gliomas (16.7%), and four WHO grade I/II gliomas (11.1%). For the remaining eight (22.2%) recurrent gliomas, histologic grade at recurrence was defined as the histologic grade at initial diagnosis. As a result, the eight recurrent gliomas were regarded as three glioblastomas, two anaplastic gliomas, and three WHO grade I/II gliomas; these grades were identical to the initial grades. No patient was upgraded based on radiologic findings.
In addition, histologic subtypes at initial diagnosis included 14 glioblastomas (38.9%), 5 anaplastic gliomas (13.8%), and 17 WHO grade I/II gliomas (47.3%). Ten patients experienced malignant transformation from low- to high-grade glioma (3 anaplastic gliomas and 7 glioblastomas). MGMT gene promoter methylation status was retrospectively examined in 15 patients (42%), with 8 exhibiting methylated MGMT.
The median time interval between initial RT and re-irradiation was 30.5 months (range, 4–173 months). Median initial RT dose was 59.4 Gy (range, 32.4–70.0 Gy) and median re-irradiation dose was 45 Gy (range, 30.0–64.6 Gy). Median cumulative doses were 100.2 Gy (range, 82.8–123.0 Gy) for the two irradiations and 195.8 Gy (range, 157.3–239.7 Gy) for median cumulative BED (α/β=2 Gy). Of the 36 patients, re-irradiation fields were local brain in 29 patients, whole brain in 5, and craniospinal irradiation in 2. Among the 29 patients who received local brain RT, 15 patients had measurable recurrent glioblastoma and the median tumor volume at recurrence of these patients was 67 cm3 (range, 10–170 cm3). Salvage surgery before re-irradiation was performed for 27 (75%) patients, consisting of gross total resection in 8 and subtotal resection in 19 patients. Among 9 patients who did not undergo surgical resection, 8 had multiple recurrent lesions, and one patient with recurrent glioblastoma diagnosed 8 months after initial RT did not undergo surgical resection due to poor performance status with KPS 30. Concurrent or sequential chemotherapy was administered to 19 (52.8%) patients with 14 receiving temozolomide (TMZ) and 5 receiving procabazine, lomustine, and vincristine. Patient and treatment characteristics are summarized in Table 1.
Median follow-up for surviving patients was 17 months (range, 12–62 months), with 32 patients died of the disease at 2–50 months after re-irradiation, 2 alive with disease at 8 and 14 months after re-irradiation, and 2 alive without disease at 14 and 62 months after re-irradiation. Median OS was 11.0 months [95% confidence interval (CI), 8.6–13.3 months] and actuarial 1-year OS rate was 41.7% (95% CI, 29.7–63.5%). PFS was 8.0 months (95% CI, 5.6–10.3 months) and actuarial 1-year PFS rate was 22.2% (95% CI, 14.4–39.1%) (Fig. 1).
Potential prognostic variables examined in univariate and multivariate analyses were gender (female vs. male), age at the time of re-irradiation (≥40 years vs. <40 years), KPS (<70 vs. ≥70), salvage surgery (no vs. yes), WHO tumor grade at recurrence (I/II vs. III vs. IV), dose of re-irradiation (≥45 Gy vs. <45 Gy), length of time interval between first RT and re-irradiation (continuous, months), and concurrent or sequential chemotherapy (no vs. yes). In univariate analysis, KPS (p<0.001), re-irradiation dose (p=0.04), and length of time between first RT and re-irradiation (p=0.04) were associated with OS (Table 2, Fig. 2). In multivariate analysis, KPS [95% CI=1.1–8.7, hazard ratio (HR)=3.1, p=0.030] and length of time between first RT and re-irradiation (95% CI=0.7–0.9, HR=0.8, p=0.048) were independent prognostic factors for survival after re-irradiation (Table 3). Of all patients, we checked out 14 patients (7 with glioblastoma, 3 with WHO grade III glioma, 4 with WHO grade I/II glioma) who survived longer than 12 months (median OS, 23.5 months) after re-irradiation. These patients had good performance status (median KPS, 80) and long time interval between RT (median time interval, 41.2 months).
We performed a specific subgroup analysis in terms of patient characteristics and survival outcome. Twenty-one patients with glioblastoma at recurrence showed a median OS of 10 months. Of these patients, 18 (86%) underwent surgery, and 9 (43%) had KPS greater than 70. KPS (<70 vs. ≥70) was independently and significantly correlated with OS among these patients (p=0.009). For the 27 patients (75%) who underwent surgical resection, median OS was 11 months. These patients had a median time interval between RT of 33 months. Among them, fifteen patients (55.6%) had a KPS greater than 70 and 18 patients (66.7%) were diagnosed with recurrent glioblastoma.
During RT, no acute toxicity ≥grade 3 occurred. The most common side effect was grade 1 or 2 headache, which was tolerable and could be relieved with medication. We evaluated toxicity in 24 patients with sufficient follow-up images and found that six patients had suspicious RN findings on MRI. However, it was not completely differentiated from tumor progression. One patient underwent salvage surgery after the 2nd recurrence, and it was histologically confirmed as glioma recurrence without RN. The remaining 5 patients (14%) were followed until death from suspected RN or recurrence (Table 4). There was a trend toward more suspicious RN (p=0.080) with a total dose of re-irradiation >40 Gy, compared to a total dose of re-irradiation ≤40 Gy on univariate analysis (17% vs. 8%). The median cumulative BED (α/β=2 Gy) of these 5 patients was 206.7 Gy (range, 181.3–232.5 Gy), and the median time between prior RT and re-irradiation was 32 months (range, 19–173 months). Neurological deterioration from suspected recurrent disease or RN was seen in 3 patients (patients 1, 2, and 3) at 3, 7, and 8 months after re-irradiation along with management with dexamethasone and anticonvulsant drugs. The remaining 2 patients (patient 4 and 5) did not show neurological deterioration.
Patients with recurrent gliomas after multimodality treatment, including RT, often have no options for effective treatment. Nonetheless, re-irradiation for gliomas is increasing in spite of possible toxicities. As most patients receive 50–60 Gy in 1.8–2 Gy per fraction, which is the tolerance dose for brain, most radiation oncologists are reluctant to use re-irradiation. In a collective review, the majority of glioma recurrences were found to occur within 2 cm of the original tumor,522 and this results in irradiation of normal brain tissue that was previously irradiated. Therefore, radiation toxicity is a concern in clinical applications of a second course of brain RT.
Studies of re-irradiation show varying toxicity results with heterogeneous patient characteristics and RT techniques including dose-fractionation, RT dose, RT volume, and concomitant chemotherapy, which are suggested as potential factors for radiation-related toxicity. Shepherd, et al.12 found that re-irradiation doses above 40 Gy for patients who received a median dose of 55 Gy (range, 45–60 Gy) as part of prior RT was a major predictor of radiation damage and observed late radiation-related damage in 13 patients (36%). In contrast, however, Gutin, et al.17 reported no clinical or radiographic RN in 25 patients with recurrent glioma who received 30 Gy of hypofractionated re-irradiation to a recurrent tumor smaller than 3.5 cm, and Fogh, et al.18 reported that only a single patient (0.6%) of 147 with recurrent malignant glioma who received a median 60 Gy dose of initial RT and a median re-irradiation dose of 35 Gy for a recurrent tumor with median volume 22 mL, experienced late grade 3 severe headaches. These reports indicate that, with the increasing conformality of RT, small volumes of irradiation can be more tolerable at higher radiation doses without RN than larger volumes. The use of additional chechemotherapy agents has survival outcomes similar to re-irradiation alone, but is associated with increased toxicity. Minniti, et al.19 reported that, of 36 patients with recurrent GBM who received 37.5 Gy of re-irradiation delivered in 15 fractions with a combination of concomitant TMZ, 3 (8%) experienced neurological deterioration from RN. In our cohort, the incidence of clinical grade 3 or 4 neurologic toxicity after re-RT was 13.8%, which was higher than other studies, although direct comparisons are impossible. However, all 5 patients had a suspected mixture of RN and tumor recurrence that could be managed with dexamethasone and anticonvulsants. Our median total re-irradiation dose of 45 Gy was higher than 40 Gy that is thought to be the limit for treating pre-irradiated patients with acceptable toxicity.12 Indeed, a trend toward more RN (p=0.080) with a total dose of re-irradiation >40 Gy was observed on univariate analysis in this study. However, like other reports, we could not identify clinically relevant factors for brain toxicity after re-RT.
Because of the lack of well-designed prospective or retrospective studies, the beneficial effects of re-irradiation for recurrent gliomas are not known. Most studies121314151617181920 included a small number of heterogeneous patients with different tumor grades and combined treatment modalities, and reported a median OS of 7 to 12 months. Median OS from the time of re-irradiation of 11 months in the current study were consistent with these outcomes. Suggested prognostic factors associated with improvement in OS were younger age,1218 higher KPS,12151920 lower histologic grade at initial diagnosis,1223 lower histologic grade at recurrence,15 longer interval between prior RT and re-irradiation, 1519 higher dose of re-irradiation,12 smaller tumor volume at re-irradiation,121318 and MGMT methylation status.19 In our single-institution patient series, KPS, dose of re-irradiation, and length of time between RT were significant potential prognostic factors influencing OS. Other potential prognostic factors including age, histology at initial presentation, histology at recurrence, and MGMT methylation did not significantly affect survival. The lack of difference in survival after re-irradiation by histologic grade at recurrence indicated that glioblastoma patients exhibit survival outcomes comparable to low-grade glioma patients. The median OS of glioblastoma versus the non-glioblastoma at recurrence was 10 months versus 12 months (p=0.958). Patients with recurrent glioblastoma were more likely to undergo surgical intervention than others (86% vs. 60%, p=0.079), and this might result in favorable outcome in glioblastoma patients. In our cohort, survival did not vary according to MGMT methylation status, probably due to small number of patients and other confounding factors affecting this study, including performance status. Patients with unmethylated MGMT (n=8) had better performance (KPS ≥70) than patients with methylated MGMT (n=7) (71.4% vs. 50%, p=0.398). Our sample size was not sufficient to detect statistical differences in survival according to previously suggested prognostic factors such as age, histology, surgical resection, and MGMT methylation status. Although we used less strict criteria for selecting patients for re-irradiation, our patients were somewhat selected. Of all patients, 31 (86%) had an interval of more than 12 months between RT, 27 (78%) were diagnosed with histologically proven recurrent glioma, and 26 (75%) underwent surgical resection. For these reasons, median OS was in line with previous report, although patients in the present study had adverse factors: 60% of all patients had glioblastoma, 20% had KPS below 40, and almost all patients with glioblastoma had a large recurrent tumor volume.
Optimal dose-fractionation for re-irradiation has not yet been well established. Conventionally fractionated RT with about 40 Gy has been most commonly used for re-irradiation of recurrent gliomas. Hypofractionated RT (HFRT) has been used for re-irradiation to reduce overall treatment time and increase tumoricidal effects, with encouraging results. Fogh, et al.18 demonstrated that HFRT delivered as 35 Gy in 10 fractions was well tolerated and resulted in a median survival time of 11 months, and Vordermark, et al.16 reported a median OS of 9.3 months and a very low rate of side effects for 19 patients with recurrent gliomas treated with HFRT delivered as 30 Gy in 6 fractions. However, as these patients had highly selected small volume tumors, the results should be interpreted cautiously.
As in most previous studies, our cohort was heterogeneous, with a small number of patients, and the results cannot directly be applied to all recurrent glioma patients. Therefore, selecting candidates for re-irradiation is a challenging process for both physicians and patients. Fundamentally, the life expectancy of the patient, the natural history of recurrent glioma, and the morbidity of treatment must all be critically examined. The fractionation scheme used in the present study was chosen for each patient, and it is difficult to suggest a standard dose fractionation schedule for recurrent gliomas in general. Nevertheless, it might be a reasonable approach to give 40–45 Gy by conventional fractionated RT for recurrent grade II or III glioma, and HFRT is worth investigating as treatment for recurrent glioblastomas that may shorten treatment time and enhance tumoricidal effects.
Another issue in applying re-irradiation is differential diagnosis with RN. In particular, for glioblastoma patients treated with RT and concurrent TMZ, we were often faced with contrast enhanced lesions, which can either be tumor recurrence or RN and are impossible to differentiate.
Therefore, we currently do not apply re-irradiation to equivocal contrast-enhanced lesions. Based upon our experiences and published studies, our institution's policy for re-irradiation of recurrent glioblastoma is as follows: 1) KPS ≥70; 2) a pathologically confirmed recurrent glioblastoma that underwent maximal surgical resection; and 3) an interval of >1 year between irradiations, as re-irradiation was unlikely to be helpful for radioresistant tumors that recurred early after initial treatment. We now apply HFRT (45 Gy for GTV and 37.5 Gy for CTV in 3 weeks) for recurrent glioblstoma patients.
In conclusion, our results showed that re-irradiation of approximately 40–45 Gy for selected recurrent glioma patients treated with 60 Gy of prior RT was feasible and had acceptable complications. Re-irradiation in conjunction with surgery could be a salvage treatment for selected recurrent glioma patients with good performance status and recurrences that occurred over a longer period of time.
Figures and Tables
 | Fig. 1Overall survival (OS) and progression-free survival (PFS) for recurrent glioma patients treated with re-irradiation. |
 | Fig. 2Overall survival for recurrent glioma patients with different Karnofsky performance status (KPS) categories who received re-irradiation. |
Table 1
Patient and Tumor Characteristics

Table 2
Univariate Analysis of Prognostic Factors in Patients Who Received Re-Irradiation

Table 3
Multivariate Analysis of Prognostic Factors in Patients Who Received Re-Irradiation
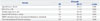
Table 4
Characteristics of Patients Who Showed Suspicious MRI Findings of Radiation Necrosis after Re-Irradiation

ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014R1A2A1A10052762).
References
1. Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989; 16:1405–1409.

2. Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009; 27:1275–1279.

3. Milano MT, Okunieff P, Donatello RS, Mohile NA, Sul J, Walter KA, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010; 78:1147–1155.

4. Dobelbower MC, Burnett Iii OL, Nordal RA, Nabors LB, Markert JM, Hyatt MD, et al. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 2011; 55:77–81.

5. Sherriff J, Tamangani J, Senthil L, Cruickshank G, Spooner D, Jones B, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013; 86:20120414.

6. Dirks P, Bernstein M, Muller PJ, Tucker WS. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993; 36:271–275.
7. Franceschi E, Omuro AM, Lassman AB, Demopoulos A, Nolan C, Abrey LE. Salvage temozolomide for prior temozolomide responders. Cancer. 2005; 104:2473–2476.

8. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007; 25:4722–4729.

9. Chinot OL, Honore S, Dufour H, Barrie M, Figarella-Branger D, Muracciole X, et al. Safety and efficacy of temozolomide in patients with recurrent anaplastic oligodendrogliomas after standard radiotherapy and chemotherapy. J Clin Oncol. 2001; 19:2449–2455.

10. Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999; 17:2572–2578.

11. Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999; 17:2762–2771.
12. Shepherd SF, Laing RW, Cosgrove VP, Warrington AP, Hines F, Ashley SE, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997; 37:393–398.

13. Hudes RS, Corn BW, Werner-Wasik M, Andrews D, Rosenstock J, Thoron L, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999; 43:293–298.

14. Arcicasa M, Roncadin M, Bidoli E, Dedkov A, Gigante M, Trovò MG. Reirradiation and lomustine in patients with relapsed highgrade gliomas. Int J Radiat Oncol Biol Phys. 1999; 43:789–793.

15. Veninga T, Langendijk HA, Slotman BJ, Rutten EH, van der Kogel AJ, Prick MJ, et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol. 2001; 59:127–137.

16. Vordermark D, Kölbl O, Ruprecht K, Vince GH, Bratengeier K, Flentje M. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005; 5:55.

17. Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009; 75:156–163.

18. Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010; 28:3048–3053.

19. Minniti G, Armosini V, Salvati M, Lanzetta G, Caporello P, Mei M, et al. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J Neurooncol. 2011; 103:683–691.

20. Niyazi M, Ganswindt U, Schwarz SB, Kreth FW, Tonn JC, Geisler J, et al. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012; 82:67–76.

21. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download