Abstract
Patients with neurofibromatosis type II will eventually succumb to bilateral deafness. For patients with hearing loss, modern medical science technology can provide efficient hearing restoration through a number of various methods. In this article, several hearing restoration methods for patients with neurofibromatosis type II are introduced.
Most patients with neurofibromatosis type II (NF-II) will eventually encounter bilateral deafness, creating an obstacle to social life. In patients with NF-II, sound amplification with hearing aids does not provide an effective solution, as speech discrimination scores (SDSs) usually remain low in the presence of retrocochlear lesions. Certain NF-II patients with mild to moderate hearing loss might benefit slightly from the use of hearing aids; however, in most cases, their hearing loss is progressive and often inevitably exceeds a level that can be rehabilitated by hearing aids.
In the past, no effective methods for rehabilitating patients with severe to profound hearing loss existed; consequently, lip-reading and sign language have been the only means of communication. In 1979, House and Hitselberger successfully performed a single-channel auditory brainstem implant (ABI) for the hearing rehabilitation of a patient with NF-II. Subsequently, the principles and concepts of treatment for patients with severe to profound hearing loss have changed.
Advancements in electronic and medical devices have provided satisfactory treatment results in patients with severe to profound hearing loss. Otologists should be fully aware of the diverse indications and potential benefits of every possible hearing rehabilitation method in order to provide active support for patients with NF-II.
NF-II is an autosomal dominant neoplastic syndrome. It has an incidence of 1 in 25000 people and a penetrance of nearly 100% at 60 years of age.1 Clinical manifestations include central and peripheral nervous system tumors, and the hallmark of NF-II is the development of bilateral vestibular schwannomas (VSs), which present in 90% to 95% of NF-II patients.2 Though VSs are benign tumors, they cause significant hearing loss, with binaural hearing loss occurring in nearly all NF-II patients. However, the mechanisms of hearing loss in NF-II are not truly understood.
The most frequent hypothesis regarding the source of hearing loss in NF-II is that it is caused by direct compression of the cochlear nerve. Previous studies have reported that the presence of hearing loss is correlated with larger tumor volumes.3 However, this hypothesis cannot explain hearing loss when tumors are small, progressive hearing loss in the presence of non-growing tumors, or unpredictable onset of hearing loss.45
In 2012, Roosli, et al.6 reviewed the histopathology of cochleae in patients with VSs and observed loss of inner and outer hair cells, atrophy of the stria vascularis, loss of cochlear neurons, and the presence of endolymphatic hydrops. The nontumorous cochleae did not present with such changes. These structural changes were not correlated with tumor size or distance from the cochlea.6 These findings indicate that hearing loss in VSs is more likely due to end organ failure than proximal cochlear nerve functions. It was recently reported that elevated levels of intralabyrinthine protein as observed via fluid attenuated inversion recovery magnetic resonance imaging (FLAIR MRI) is closely associated with hearing loss in NF-II.3 Intralabyrinthine protein elevation is caused by cochlear aperture obstruction or destruction by VSs and explains the end organ failure in NF-II. It was also demonstrated that increases in intralabyrinthine protein obtained from perilymph aspiration could accurately identify the presence of VSs.7
The pattern of hearing loss is unpredictable in NF-II. Sudden, relapsing, or progressive hearing loss may occur regardless of tumor size or time from initial diagnosis. However, the natural history of hearing loss in NF-II has previously been studied.4 In this study, 108 ears were examined; the pure-tone average (PTA) at baseline was 22.2±21.8 dB with a mean SDS of 93.9±13.1%. Without treatment, the follow-up PTA at 2 years decreased to 37.0±31.4 dB, and SDS also dropped to 83.9±28.4%. NF-II is likely to be diagnosed before any loss of hearing, indicating that in most cases, the baseline hearing is normal. This decrease is faster and more severe than that of general age-related hearing loss,8 and of note, a decrease in SDS severely reduces the effectiveness of hearing aids. While hearing aids can be helpful for any patient with hearing loss, those with moderate levels of loss receive the most potential benefit. Severe or profound loss can limit the usefulness of even the most powerful hearing aids. In many cases of profound hearing loss, a hearing aid can only provide environmental awareness or a degree of rudimentary perception of speech.9
Contralateral routing of signals (CROS) hearing aids have been introduced to provide audiologic benefits by routing the sound from the hearing-impaired side to the intact ear. Use of CROS hearing aids is the easiest method for rehabilitation in patients with asymmetric hearing loss without surgery; however, there are several limitations, including poor cosmetics due to the use of two hearing aids with a connection wire, discomfort from the occlusion of the better ear, and most of all, degradation of speech intelligibility in certain demanding situations10 and significant deficit in noisy environments.11 Despite its convenience and simplicity, cosmetic inferiority and discomfort from the occlusion effect in the normal ear has precluded the widespread use of CROS as a conventional type of hearing aid.
For patients with single-sided deafness, occurring either spontaneously or after tumor removal, a bone-conduction hearing implant (BCHI) can be a good hearing rehabilitation option. In this option, the speech processor receives the auditory signal and transforms it to a vibration, after which the BCHI transmits the vibration to both the ipsilateral and contralateral cochleae as it passes through the skin and skull. Conductive hearing loss, mixed hearing loss, and single-sided deafness are all good candidates for BCHI. In cases of patients with single-sided deafness, the vibration generated from the deaf side is transferred to the contralateral normal cochlea, where it can be recognized. However, BCHI is not as applicable to binaural hearing, as it does not offer many of the advantages of binaural hearing: summation effects, binaural squelch, and sound localization cannot be attained (Fig. 1). Despite these limitations, patients' satisfaction with BCHI is usually quite high.1213
From its first introduction in 1976,14 BCHIs have developed at a remarkable pace, and various products have been commercialized or are in clinical trials. BCHIs can be classified according to transmit system: one is a direct-drive system that transmits the vibration through the skull, and the other is a skin-drive system that transmits the vibration through the skin (Fig. 2).
Examples of direct-drive systems include Ponto® (Oticon Medical, Smørum, Denmark), Baha® BP100 (Cochlear Bone Anchored Solutions AB, Molnlycke, Sweden), and Bonebridge™ (MED-EL, Innsbruck, Austria). The Ponto® and Baha™ BP100 systems use screws to attach the speech processor to the skull, so that the vibrations generated from the device are directly transmitted to the skull. They are known to achieve a sufficient hearing gain, as the vibration directly transmits via the screw, and a degree of binaural squelch effect has also been reported.15 Despite the above-mentioned benefits, recipients often suffer from various skin problems and loosening of the screws due to their fixation methods.16 To overcome these limitations, active transcutaneous devices were introduced. In the case of the Bonebridge™ system, active BCHIs are implanted into the temporal bone and vibrate within the temporal bone. Speech processors are attached to the scalp through magnets. This promotes sufficient hearing gain and is not associated with any skin problems. However, larger internal devices are required, which has made it difficult to perform such implantations in children or in patients who have undergone previous mastoidectomy. Various methods are followed to overcome these limitations, such as the use of a lift system or the retrosigmoid approach (RSA).17
A skin-drive system generates the vibration outside of the skull. The vibration must pass through the skin to reach the skull. Softband Baha® (Cochlear Bone Anchored Solutions AB, Molnlycke, Sweden), Sophono® (Medtronic, Louisville, CO, USA), and Baha® Attract (Cochlear Bone Anchored Solutions AB, Molnlycke, Sweden) use this system. Softband Baha® uses elastic headbands around the head, instead of screws, to fix the device to the skull. It can be used with children who are too young to undergo implant surgery.18 In addition, it is quite effective at simulating and predicting the outcomes of BCHIs and can thus be used with patients who are planning to undergo BCHI surgeries.19 However, for secure fixation, the pressure that is required may cause deformations of the skin and subcutaneous tissues, and tension headaches. This problem can be solved by using magnets, which is how the speech processors of Sophono® and Baha® Attract are attached to the scalp. However, thick skin could weaken the magnetic forces and reduce the fixation; thus, the thickness of skin should be less than 5 mm.20 A skind-rive system tends to have less hearing gain than a direct-drive system due to the attenuation of the mechanical energy as it passes through skin;21 however, theoretically it can be overcome by fixing the speech processor in the proper location with adequate pressure.22
As all BCHIs contain permanent magnets, MRI compatibility is a challenging concern. Computed tomography (CT) should be used as a first choice for the safety of both patients and devices; however, certain clinical conditions require an MRI scan in order to obtain proper image information. In cases of NF-II, regular MR imaging is required. Bonebridge™ and Sophono® are certified to be conditionally safe up to 1.5 and 3 Tesla,23 respectively, although there will be an image artifact in the region near the implant. Manufacturers have specified the size of the corresponding MRI artifact, specifically, a sphere of 15 cm in diameter for Bonebridge™, a distance of 5–10 cm from the Sophono® implant, and a distance of 11.5 cm from the center of the Baha® Attract implant.2425 Steinmetz, et al.26 also reported a 29-year-old patient with VS who was scanned with a Bonebridge™ implant on the contralateral side; due to the image artifact, the tumor was concealed. If an MRI absolutely needs to be performed with a focus on structures in the skull near the implant, the implant must be explanted to eliminate artifacts. Therefore, in patients with NF-II, it is important to select the proper implant for such an event.
In the early days of their use, patients with ABIs were only able to recognize environmental sounds or to detect sounds with a further reliance on lip-reading, and they were not capable of discriminating speech sounds.27 For better hearing rehabilitation, a cochlear implant (CI) is applied to patients with NF-II. CIs offer many advantages over ABIs, and the most important benefit is that CIs can provide better speech understanding. As intracochlear electrode placement permits reliable tonotopic stimulation, better auditory performance is generally expected.28 A CI can be attempted either with the tumor remaining in place or after the tumor has been removed.
Two decades ago, the first CI was performed simultaneously with the removal of a tumor in a patient with NF-II.29 CI was also attempted on patients who were non-surgically treated, such as those who underwent stereotactic radiotherapy [or a gamma knife surgery (GS)] or refused to undergo surgery. GS is an alternative to surgery for tumor control and has been proposed to allow hearing preservation, though it is not without risks. Prasad, et al.30 reported the outcomes of 200 cases of VSs that were treated with GS, and 25% demonstrated either an increase or no changes of volume, while hearing deterioration was found in 60% of the patients over a 6-year period. Outcomes for CI after GS for VS are varied in that some were able to achieve good post-implantation speech perception, while others were only capable of detecting environmental sounds.31 However, regardless of the variable outcomes, the problem is that tumors can grow at any time without complete tumor removal, and larger tumors are correlated with hearing loss.3 Additionally, the function of CI after GS for VS is likely to decrease over time, and malignant transformation is also a concern. There have been reported cases of malignant transformation of tumors in NF-II following GS, and reports suggest that up to 50% of all malignant transformations occur in NF-II patients.32 It is important to remember that regular follow-up via MRI is critical when patients are non-surgically treated. If such imaging is obtained with the magnetic device implanted, many adverse effects could occur. MRI performed on patients with an implanted magnetic device could potentially result in a migration of the device. Demagnetization and malfunction of the devices could also occur, and the heating of such internal devices could damage the surrounding tissues. These effects can be avoided by removing the magnets before performing MRI scans or by using low-Tesla (T) MRI. Current US Food and Drug Administration (FDA) guidelines have approved the use of 0.2 to 1.5 T with the magnet in place.33 Generally, MRI in patients with a magnetic device is reported to be safe with elastic head bands placed around their heads.34 Furthermore, heat from the CI during 1.5-T MRI is reported to be lower than 0.1℃;34 hence, usage of 1.5-T MRI with the magnetic device is relatively safe. However, image quality obtained from the MRI scan is another issue. Ipsilateral soft tissues within 7 to 8 cm from the magnet are poorly visualized, while the contralateral side image has no distortion.35 Several otologists recommend simultaneous high-resolution computed tomography (HRCT) imaging and a comparison of the images for better expression.
If CI is to be performed simultaneously with tumor removal, it is necessary to preserve the cochlear nerve while removing the tumor. VSs mostly originate from vestibular nerves; therefore, preserving the cochlear nerve while removing the tumor is possible in certain cases.36 Sporadic VSs tend to grow and only compress the nearby cochlear nerve. However, VSs in patients with NF-II directly invade into the cochlear nerve37 and are quite adherent to the cochlear nerve, making safe dissection of the cochlear nerve from the tumor difficult.29 Although anatomically well-separated, histological injuries such as minor bleeding or an axonal injury might decline the function of the cochlear nerve.38 However, though an injured cochlear nerve cannot function with the auditory signal, the nerve can function with a direct electrical signal under the conditions of the CI. Response to an electrical signal can be evaluated by stimulating the cochlear promontory with an electrode during the operation.
Various approach techniques can be applied; however, a translabyrinthine approach (TLA) is known to be the best approach for accomplishing both complete tumor removal and successful CI. TLA, which provides the otologist with a familiar surgical view, offers early identification of the cochlear nerve in the auditory canal during the surgery and eliminates any need for cerebellar retraction.39 Cole, et al.40 compared the RSA with TLA for VS resection and observed that TLA was associated with a lower risk of cochlear nerve injury, which is essential for performing CI, dysphagia, and dysrhythmia.
There are many reports of performing CIs after tumor removal in either sporadic or NF-II-associated VSs.3141424344 Generally, outcomes for sound field and speech perception in post-lingual CI patients and in patients with tumors have been similar, and half of the patients with tumors were able to communicate on telephones.45 It is not certain what the appropriate time interval is between implantation and tumor removal. Theoretically, simultaneous implantation would reduce the possibility of failure, as the chance of fibrosis or ossification of the cochlea could be avoided. Furthermore, distortion of the anatomy due to tumor removal can be minimized if the implantation is performed simultaneously. The time required for fibrosis or ossification of the cochlea to occur is unknown; however, there have been reports of CI failure occurring due to cochlear ossification when performed 1 year after removing a tumor via TLA.43 On the other hand, there is also a report of a successful implantation being performed 3 months after the tumor removal, via the same approach.42
After the first successful implantation in 1979, ABIs were originally the only way to restore hearing in patients with NF-II. Initially, ABIs were developed for patients who could not benefit from a CI, i.e., patients with non-functioning cochlear nerves. Patients with NF-II are typical candidates; however, the indications of ABI are becoming wider, such that patients with a cochlear anomaly or a cochlear ossification following meningitis are now also good candidates.46
The operation principle of an ABI is similar to that of a CI. In cases of CIs, the external auditory processor receives the auditory signal and transforms it into an electrical signal. This signal is then transmitted to the electrode that was inserted into the cochlea, and the stimulation is delivered to the auditory nerve. Finally, the auditory signal is recognized in the brain. In cases of ABIs, the electrode is inserted in the cochlear nucleus, which is located at the brainstem, proximal to the auditory nerve. The transformed signal is then transmitted to the electrode, and the brain recognizes the signal (Fig. 3).
The cochlear nucleus is located on the dorsal lateral side of the lateral recess of brainstem and can be accessed via the Foramen of Luschka. For successful implantation, inserting the electrode into the exact location of the cochlear nucleus is key to the procedure. However, detecting the exact location is difficult, as these structures are covered with flocculus and are not easily observed in a natural state due to the occurrence of anatomical distortions from either the tumor itself or the operation. TLA provides the otologist with a familiar and direct surgical view, providing a solution to this anatomical problem. Furthermore, TLA is free from cerebellar retraction and is advantageous when performing ABI. A total of 90% to 95% of cases of NF-II have bilateral VSs.2 If a contralateral VS is too large, cerebellar retraction is hardly feasible; thus, RSA would not be able to provide the proper surgical views. Moreover, VSs in NF-II are likely to grow into the internal auditory canal,47 preventing complete tumor resection via RSA (Fig. 4). For these reasons, TLA is recommended by experienced surgeons.48 Intra-operative monitoring is also strongly recommended for better detection of the exact implant location.49
ABIs provide effective hearing rehabilitation for patients who cannot benefit from CI. Since the first ABI in 1979, House Ear Institute (Los Angeles, CA, USA) surgeons have performed more than 230 ABIs. In their patient series, 85% were able to detect the auditory signal, and 93% showed great improvement in understanding sentences with the assistance of lip-reading.27 In 2002, two implantees were reported to have the ability to communicate on the phone.50 Vincenti, et al.43 compared the outcomes of hearing rehabilitation in patients with NF-II who underwent tumor removal and received either a CI or ABI at a single institute. In closed-set conditions, patients with a CI showed outstanding results compared to patients with an ABI. However, in open-set conditions, the two groups did not show significant differences. CIs in cases of NF-II need to preserve the cochlear nerve while completely removing the tumor, which is a difficult task. Remaining tumor tissue lowers the CI function and makes it difficult to follow-up via MRI. With ABIs, on the other hand, it is possible to perform the surgery without preserving the cochlear nerve, making it easier to completely remove the tumor and follow-up via MRI.
VSs, especially in cases of NF-II, were once considered to be a life-threatening disease. However, with early diagnosis, treatment with a multidisciplinary approach, and advancements in electronic and medical devices, not only have survival rates improved but the quality of life has also substantially been enhanced with options for successful hearing rehabilitation.
Figures and Tables
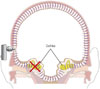 | Fig. 1Mechanisms of a bone-conduction hearing implant. Vibrations generated from the device are transferred to the contralateral cochlea and recognized on the contralateral side. |
 | Fig. 2Categorization of bone-conduction hearing implants. (A) Ponto®. (B) Baha® BP100. (C) Bonebridge™. (D) Softband Baha®. (E) Baha® Attract. (F) Sophono®. Photos provided courtesy of Oticon Medical (A), Cochlear Bone Anchored Solutions AB (B, D, and E), MED-EL (C), and Medtronic (F). |
 | Fig. 3Concurrent tumor removal and auditory brainstem implant via the translabyrinthine approach. (A) Implantable internal device. (B) Diagram of auditory brainstem implant via translabyrinthine approach. The tumor, which originated from the cochleovestibular nerve, was resected with the nerve. A flat electrode was inserted, which stimulated the dorsal cochlear nucleus. Photos provided courtesy of Cochlear Headquarters (A). The figure was created by Dong-Su Jang, medical illustrator (B). |
 | Fig. 4Comparison of the retrosigmoid approach and the translabyrinthine approach for tumor removal. (A) Severe cerebellar retraction is needed to expose the tumor; however, tumors in the internal auditory canal are not readily removable via the retrosigmoid approach. (B) In the translabyrinthine approach, tumors in the internal auditory canal are well exposed without cerebellar retraction. Arrows indicate the direction of visual field. |
ACKNOWLEDGEMENTS
The authors would like to thank Dong-Su Jang, MFA (Medical Illustrator, Medical Research Support Section, Yonsei University College of Medicine, Seoul, Korea), for his help with the illustrations.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korean government (2014R1A1A2058141).
References
1. Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005; 26:93–97.

2. Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992; 84:603–618.
3. Asthagiri AR, Vasquez RA, Butman JA, Wu T, Morgan K, Brewer CC, et al. Mechanisms of hearing loss in neurofibromatosis type 2. PLoS One. 2012; 7:e46132.

4. Masuda A, Fisher LM, Oppenheimer ML, Iqbal Z, Slattery WH. Natural History Consortium. Hearing changes after diagnosis in neurofibromatosis type 2. Otol Neurotol. 2004; 25:150–154.

5. Graamans K, Van Dijk JE, Janssen LW. Hearing deterioration in patients with a non-growing vestibular schwannoma. Acta Otolaryngol. 2003; 123:51–54.

6. Roosli C, Linthicum FH Jr, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012; 33:473–480.

7. Silverstein H. Labyrinthine tap as a diagnostic test for acoustic neurinoma. Otolaryngol Clin North Am. 1973; 6:229–244.

8. Gates GA, Cooper JC Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983-1985. Part I. Basic audiometric test results. Ear Hear. 1990; 11:247–256.
9. Flint PW, Haughey BH, Lund V, Niparko JK, Robbins KT, Thomas JR, et al. Cummings otolaryngology. Philadelphia, PA: Elsevier;2015.
10. Lotterman SH, Kasten RN. Examination of the CROS type hearing aid. J Speech Hear Res. 1971; 14:416–420.

11. Lin LM, Bowditch S, Anderson MJ, May B, Cox KM, Niparko JK. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol. 2006; 27:172–182.

12. Laske RD, Röösli C, Pfiffner F, Veraguth D, Huber AM. Functional results and subjective benefit of a transcutaneous bone conduction device in patients with single-sided deafness. Otol Neurotol. 2015; 36:1151–1156.

13. Snapp H, Angeli S, Telischi FF, Fabry D. Postoperative validation of bone-anchored implants in the single-sided deafness population. Otol Neurotol. 2012; 33:291–296.

15. Kompis M, Kurz A, Pfiffner F, Senn P, Arnold A, Caversaccio M. Is complex signal processing for bone conduction hearing aids useful? Cochlear Implants Int. 2014; 15:Suppl 1. S47–S50.

16. Dun CA, Faber HT, de Wolf MJ, Mylanus EA, Cremers CW, Hol MK. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol. 2012; 33:192–198.

17. Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J. Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx. 2014; 41:84–87.

18. Hol MK, Cremers CW, Coppens-Schellekens W, Snik AF. The BAHA Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol. 2005; 69:973–980.
19. Doshi J, McDermott AL. Bone anchored hearing aids in children. Expert Rev Med Devices. 2015; 12:73–82.

20. Siegert R. Partially implantable bone conduction hearing aids without a percutaneous abutment (Otomag): technique and preliminary clinical results. Adv Otorhinolaryngol. 2011; 71:41–46.

21. Heywood RL, Patel PM, Jonathan DA. Comparison of hearing thresholds obtained with Baha preoperative assessment tools and those obtained with the osseointegrated implant. Ear Nose Throat J. 2011; 90:E21–E27.

22. Kurz A, Flynn M, Caversaccio M, Kompis M. Speech understanding with a new implant technology: a comparative study with a new nonskin penetrating Baha system. Biomed Res Int. 2014; 2014:416205.

23. Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 2015; 8:79–93.

24. Jansson KJ, Håkansson B, Reinfeldt S, Rigato C, Eeg-Olofsson M. Magnetic resonance imaging investigation of the bone conduction implant - a pilot study at 1.5 Tesla. Med Devices (Auckl). 2015; 8:413–423.
25. Rha MS, Jeong SW, Seo YW, Moon IS. Hearing rehabilitation with Sophono® in patients with unilateral hearing loss after meningioma removal. Korean J Otorhinolaryngol-Head Neck Surg. 2015; 58:514–519.

26. Steinmetz C, Mader I, Arndt S, Aschendorff A, Laszig R, Hassepass F. MRI artefacts after Bonebridge implantation. Eur Arch Otorhinolaryngol. 2014; 271:2079–2082.

27. Toh EH, Luxford WM. Cochlear and brainstem implantation. 2002. Neurosurg Clin N Am. 2008; 19:317–329. vii.
28. Carlson ML, Breen JT, Driscoll CL, Link MJ, Neff BA, Gifford RH, et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol. 2012; 33:853–862.
29. Hoffman RA, Kohan D, Cohen NL. Cochlear implants in the management of bilateral acoustic neuromas. Am J Otol. 1992; 13:525–528.
30. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2013; 119:Suppl. 745–759.

31. Lustig LR, Yeagle J, Driscoll CL, Blevins N, Francis H, Niparko JK. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006; 27:512–518.

32. Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006; 43:289–294.

33. Majdani O, Leinung M, Rau T, Akbarian A, Zimmerling M, Lenarz M, et al. Demagnetization of cochlear implants and temperature changes in 3.0T MRI environment. Otolaryngol Head Neck Surg. 2008; 139:833–839.

34. Crane BT, Gottschalk B, Kraut M, Aygun N, Niparko JK. Magnetic resonance imaging at 1.5 T after cochlear implantation. Otol Neurotol. 2010; 31:1215–1220.

35. Kim BG, Kim JW, Park JJ, Kim SH, Kim HN, Choi JY. Adverse events and discomfort during magnetic resonance imaging in cochlear implant recipients. JAMA Otolaryngol Head Neck Surg. 2015; 141:45–52.

36. Kim JW, Moon IS. Simultaneous translabyrinthine tumor removal and cochlear implantation in vestibular schwannoma patients. Yonsei Med J. 2016; In press.

37. Linthicum FH Jr, Brackmann DE. Bilateral acoustic tumors. A diagnostic and surgical challenge. Arch Otolaryngol. 1980; 106:729–733.

38. Lambert PR, Ruth RA, Thomas JF. Promontory electrical stimulation in postoperative acoustic tumor patients. Laryngoscope. 1992; 102:814–819.

39. Chamoun R, MacDonald J, Shelton C, Couldwell WT. Surgical approaches for resection of vestibular schwannomas: translabyrinthine, retrosigmoid, and middle fossa approaches. Neurosurg Focus. 2012; 33:E9.

40. Cole T, Veeravagu A, Zhang M, Azad T, Swinney C, Li GH, et al. Retrosigmoid versus translabyrinthine approach for acoustic neuroma resection: an assessment of complications and payments in a longitudinal administrative database. Cureus. 2015; 7:e369.

41. Neff BA, Wiet RM, Lasak JM, Cohen NL, Pillsbury HC, Ramsden RT, et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007; 117:1069–1072.

42. Arístegui M, Denia A. Simultaneous cochlear implantation and translabyrinthine removal of vestibular schwannoma in an only hearing ear: report of two cases (neurofibromatosis type 2 and unilateral vestibular schwannoma). Otol Neurotol. 2005; 26:205–210.

43. Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: cochlear versus auditory brainstem implantation. Audiol Neurootol. 2008; 13:273–280.

44. Tran Ba Huy P, Kania R, Frachet B, Poncet C, Legac MS. Auditory rehabilitation with cochlear implantation in patients with neurofibromatosis type 2. Acta Otolaryngol. 2009; 129:971–975.

45. Celis-Aguilar E, Lassaletta L, Gavilán J. Cochlear implantation in patients with neurofibromatosis type 2 and patients with vestibular schwannoma in the only hearing ear. Int J Otolaryngol. 2012; 2012:157497.

46. Choi JY, Song MH, Jeon JH, Lee WS, Chang JW. Early surgical results of auditory brainstem implantation in nontumor patients. Laryngoscope. 2011; 121:2610–2618.

47. Bernardeschi D, Peyre M, Collin M, Smail M, Sterkers O, Kalamarides M. Internal auditory canal decompression for hearing maintenance in neurofibromatosis type 2 patients. Neurosurgery. 2015; 11. 16. DOI: 10.1227/neu.0000000000001125. [Epub].

48. Monsell EM, McElveen JT Jr, Hitselberger WE, House WF. Surgical approaches to the human cochlear nuclear complex. Am J Otol. 1987; 8:450–455.
49. Waring MD. Intraoperative electrophysiologic monitoring to assist placement of auditory brain stem implant. Ann Otol Rhinol Laryngol Suppl. 1995; 166:33–36.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download