Abstract
Purpose
In this study, we evaluated the long term beneficial effect of Renin-Angiotensin-Aldosterone System (RAAS) blockade therapy in treatment of Marfan aortopathy.
Materials and Methods
We reviewed Marfan syndrome (MFS) patients who underwent aortic root replacement (ARR) between January 1996 and January 2011. All patients were prescribed β-blockers indefinitely. We compared major aortic events including mortality, aortic dissection, and reoperation in patients without RAAS blockade (group 1, n=27) to those with (group 2, n=63). The aortic growth rate was calculated by dividing the diameter change on CT scans taken immediately post-operatively and the latest scan available.
Results
There were no differences in clinical parameters except for age which was higher in patients with RAAS blockade. In group 1, 2 (7%) deaths, 5 (19%) aortic dissections, and 7 (26%) reoperations occurred. In group 2, 3 (5%) deaths, 2 (3%) aortic dissections, and 3 (5%) reoperations occurred. A Kaplan-Meier plot demonstrated improved survival free from major aortic events in group 2. On multivariate Cox, RAAS blockade was an independent negative predictor of major aortic events (hazard ratio 0.38, 95% confidence interval 0.30-0.43, p=0.002). Mean diameter change in descending thoracic and supra-renal abdominal aorta was significantly higher in patients without RAAS blockade (p<0.05).
Marfan syndrome (MFS) is an autosomal dominant connective tissue disorder usually caused by a mutation in the FBN1 gene that encodes fibrillin-1. Fibrillin-1 regulates TGF-β signaling, in addition to being a major structural component of extracellular matrix microfibrils.1234 Fibrillin-1 is important in multiple organ systems, including the musculoskeletal, ocular, pulmonary, cardiovascular, and central nervous systems. Defects in the cardiovascular system cause death in MFS patients. Early detection of MFS-related cardiovascular disease and prophylactic surgery have been shown to increase life expectancy up to 60 years old.567 The progressive aortic dilatation process continues even after aortic surgery, necessitating long-term medical therapy.89 β-blockers are known to slow the rate of aortic dilatation by reducing aortic wall stress through their negative chronotropic effect. This is the only medication currently proven in prospective randomized settings to reduce aortic dilatation and clinical events, and to improve survival.10 However, about one out of ten MFS patients who underwent aortic surgery experiences re-operation in spite of continuous β-blocker therapy.111213
Previous researches have shown that propranolol has no beneficial effects on elastic fiber fragmentation or aortic wall structure, whereas losartan-treated mice show definite improvement in both parameters.14 In a non-randomized, retrospective study involving 18 pediatric MFS patients who had prominent aortic dilatation despite β-blocker therapy, the addition of an angiotensin receptor blocker (ARB) clearly reduced the rate of aortic root dilatation.15 Moreover, angiotensin converting enzyme inhibitors (ACEIs) also reduced aortic stiffness and dilatation in MFS patients.1617 However, long-term data supporting the efficacy of Renin-Angiotensin-Aldosterone System (RAAS) blockade in combination with β-blockers in MFS patients who have undergone aortic root replacement is lacking. Therefore, we evaluated the long term beneficial effect of RAAS blockade therapy in treatment of Marfan aortopathy.
This was a retrospective study of patients seen at a 2000-bed tertiary teaching hospital. The study protocol was approved by the Institutional Review Board of Severance Cardiovascular Hospital (# 4-2013-0524) and was in compliance with the Declaration of Helsinki. Between January 1996 and January 2011, using ICD-9 codes, we identified 143 consecutive patients diagnosed with MFS (code 759.82) who had undergone aortic root replacement (ARR) operation with continuous β-blocker therapy after the operation. All patients met the revised Ghent criteria7 and had pathology consistent with medial degeneration. Patients with the diagnosis of Loeys-Dietz syndrome or Ehlers-Danlos syndrome were not included in this study. Of the 143 patients, we excluded 22 patients with prior aortic dissection, 4 patients who underwent ARR under the age of 18 years, 11 patients who had a change in β-blocker or RAAS blockade prescription status, 2 patients with mental retardation (due to the concern of uncertain drug compliance), 4 patients who were pregnant during follow up, 4 patients with more than 1 graft, 3 patients who had overlapping of Dacron graft with pulmonary artery (PA) bifurcation, and 3 patients with insufficient clinical data. As a result, we enrolled 90 MFS patients who underwent ARR in our study. Of these patients, 62 had Bentall composite graft and 28 had underwent valvesparing ARR.
Patient medical records were reviewed for information on patient age at the time of operation, gender, weight, height, blood pressure, heart rate, drug therapy, echocardiographic results and computed tomography scan results. Patient databases were searched to identify known or putative risk factors for hypertension, dyslipidemia, atrial fibrillation (AF), congestive heart failure, congenital heart disease (CHD) other than MFS, and smoking status. AF was documented with 12-lead electrocardiography or 24 hr Holter recording. Heart failure was defined as satisfying the previously published Framingham criteria18 (presence of 2 major or 1 major with 2 minor criteria). CHD included repaired or non-cyanotic atrial septal defect, ventricular septal defect, tetralogy of Fallot, or coarctation of the aorta. We analyzed heart rate and blood pressure, which were averaged with more than ten measurements at post-operative outpatient visit.
We evaluated major aortic events including all-cause mortality, newly developed aortic dissection, and reoperation for recurrent valvular regurgitation or progression distal aorta pathology. Indications for the reoperation consisted of recurrent aortic regurgitation of G3 (central jet width/LVOT width=0.50, n=1), progressive aneurysmal enlargement of the downstream aorta greater than 55 mm in 2 patients, rapidly expanding aneurysm (>5 mm in 6 months) in 6 patients, and impending aortic rupture in 2 patients. Follow-up was censored after the first aortic event. Patients who were alive and aortic event free at last follow-up were censored.
The maximum aortic diameters were measured at five levels of the aorta preoperatively; 1) ascending and 2) descending thoracic aorta (DTA) at the level of PA bifurcation, 3) aortic arch, 4) the mid-section between the diaphragm neck and right renal artery ostium, and 5) the inferior mesenteric artery (IMA) ostium level. Pulmonary artery bifurcation level corresponded to a level above Dacron graft in all patients. Imaging studies included CT scan, trans-esophageal echocardiography, magnetic resonance imaging or angiography performed within 3 months before ARR. In case of patients who were evaluated their aortic dimension by two or more modalities, we selected the maximal diameter.
Postoperative aortic growth rate of each group was compared in patients who have undergone repeated CT scanning after the operation [n=19 (70%) for group 1 vs. n=43 (68%) for group 2]. All of these patients took the first scan one week after the operation and another follow-up scan at least 1 year after operation. We compared the change in the maximal aortic diameter perpendicular to the aortic centerline at 3 levels of the aorta. The measurements were obtained from cranial to caudal at the 1) PA bifurcation level, 2) the mid-section between the diaphragm neck and right renal artery ostium, and 3) the IMA ostium level. The rate of aortic diameter change was calculated by dividing the increment of the aortic maximal diameter by the time interval on the CT scans. All collected imaging data were read in consensus by 2 radiologists who were blinded to the patients' clinical data.
Continuous variables that were normally distributed were reported as mean±SD, and were compared using Student's t-tests for parametric data and Mann-Whitney tests for nonparametric data. Categorical variables were reported as counts (percentages) and compared using chi-squared or Fisher's exact tests. Kaplan-Meier survival curves were plotted for β-blocker-only patients (group 1) or RAAS blockade-combination patients (group 2) and compared by means of the log-rank test. To identify independent predictor variables, we performed multivariate Cox proportional-hazards analysis using the factors predictive in univariate analysis (p<0.05). The Wilcoxon signed rank test was used to compare the rates of aortic diameter change in patients with or without RAAS blockade. A p-value<0.05 was considered statistically significant. SPSS statistical package (SSPS Inc., Chicago, IL, USA) version 18.0 was used to perform all statistical analysis.
Clinical characteristics and echocardiographic parameters are presented in Table 1. Of the 90 patients, group 1 patients (n=27) had no history of RAAS blockade prescription and group 2 patients (n=63) were prescribed β-blocker and RAAS blockade concomitantly after ARR. The rationale for prescription of RAAS blockade in group 2 patients according to chart review are as follows; control of blood pressure (n=22), reduced left ventricular systolic function (n=15), attendant's clinical decision (n=14), and not clearly documented on the chart (n=12). Of the 90 patients with MFS, 60 patients (67 percent) were male. There were no differences in the clinical characteristics of the two groups, except for age at the time of ARR. On average, group 2 patients were 4.4 years older than group 1 patients (34.8 years vs. 39.2 years; p=0.001). In family history, there was no difference in the presence of aortic dissection, rupture, sudden death or FBN1 mutation. There were no differences in echocardiographic parameters including aortic annulus diameter, sinus of Valsalva diameter, or ejection fraction. Mean systolic blood pressure and heart rate were not different between the two groups. The frequency of other prescription medications, including statins, digoxin, calcium channel blockers, and diuretics, were not significantly different between the two groups. More detailed demographic data that subdivided group 2 into patients with ACEI and ARB are shown in Supplementary Table 1 (only online). There was no difference in the preoperative aortic dimension between the two groups, except for the supra-renal abdominal aorta, which was higher in group 2 (Table 2). We evaluated the preoperative aortic dimension within 3 months before ARR in 24 patients (89%) of group 1 and 58 patients (92%) of group 2. There was no difference in the baseline aortic dimensions between the groups, except for the supra-renal abdominal aorta level, which was higher in patients with RAAS blockade (p=0.002).
The incidence rates of major aortic events are presented in Table 3. During the mean 82±60 months of follow-up, 2 (7%) and 3 (5%) patients died of cardiac or non-cardiac causes in group 1 and 2, respectively (p=0.32). The development of aortic dissection was significantly higher in group 1 (19% vs. 3%, p=0.02). Preoperative maximal diameters of distal aorta of the patients who developed new aortic dissection were 45.9 mm; 43.6 mm; 42.1 mm; 38.6 mm; 36.3 mm in group 1 and 44.2 mm; 37.0 mm in group 2, respectively. Of those 7 patients who developed new aortic dissection, 2 patients (40%) of group 1 and 1 patient of group 2 (50%) had positive family history of aortic dissection. The risk of re-operation for recurrent aortic regurgitation or distal aortic pathology was also higher in group 1 (p=0.003), especially at the aortic arch or DTA level (p=0.01). Consequently, there was a significant difference in the incidence of major aortic events between the two groups (p=0.001). In Kaplan-Meier analysis, group 2 patients had higher cumulative event-free survival than group 1 (p=0.008) (Fig. 1). Clinical outcome of patients treated with ACEI or ARB were not different, which was significantly better than that of group 1 patients (Supplementary Fig. 1, only online). We used univariate and multivariate Cox regression modeling to test the ability of potential baseline risk factors to predict major aortic events. Table 4 shows several variables that were associated with a higher risk of major aortic events. RAAS blockade therapy, in addition to β-blocker was an independent negative predictor of major aortic events after adjustment for age, smoking and descending thoracic aorta diameter (hazard ratio=0.38, 95% confidence interval 0.30-0.43, p=0.002).
A total of 62 patients (69%) had a CT scan performed more than twice after ARR: 19 (70%) in group 1 and 43 (68%) in group 2. Rates of aortic diameter change measured at 3 positions are depicted in Fig. 2. At the pulmonary artery bifurcation level, the growth rate was 2.7±1.4 mm/yr in group 1 and 1.1±0.7 mm/yr in group 2 (p<0.001). At the mid-section between the diaphragm and the right renal artery ostium level, the growth rate was 2.5±1.0 mm/yr in group 1 and 0.9±0.7 mm/yr in group 2 (p<0.001). There was no significant difference in the rate of aortic growth at the IMA ostium level between the groups (0.9±0.6 mm/yr in group 1 and 0.8±0.6 mm/yr in group 2, p=0.73).
Although β-blockers have been proven to decrease the rate of aortic root dilatation and reduce the risk of clinical events by reducing tensile stress on the aorta, the risk of aortic events or re-operation is still high. This is particularly true in MFS patients who have already undergone aortic operation.1920 Current guidelines on medical treatment after aortic surgery are rare and not based on randomized clinical trials.2122 RAAS blockade are attracting much attention in MFS due to their ability to modulate excessive TGF-β signaling. There are several experimental evidences that supports the superiority of ARB, selective angiotensin II receptor type (AT) I blocker, compared with non-selective angiotensin receptor blocking ACEI, as the downstream signaling of AT2 ameliorated aneurysm progression in rodent Marfan model. However, some controversy exists about the beneficial effect of AT2, as it can also induce vascular smooth muscle cell apoptosis which is prone to progression of aneurysm.23 Until now, there is no solid evidence that supports the superior efficacy of ARB compared with ACEI, especially in MFS patients who have replaced their aortic root. In this study, both ACEIs and ARBs were found to be associated with reducing aortic dilatation and major aortic events in Marfan aortopathy patients who were previously on β-blockers. This phenomenon was independent of the blood pressure reduction that can be explained by these molecular mechanisms, which are supported by recent evidence in human and animal studies.242526
In addition to its molecular mechanism, RAAS blockade is expected to have beneficial effects on Marfan aortopathy by reducing hemodynamic stress. In MFS patients, aortic stiffness increases and distensibility decreases over time.2728 In a previous study, decreased aortic distensibility was an independent predictor of progressive aortic dilatation in MFS patients.29 Early reflection of the pressure wave from the stiffened peripheral arteries increases load on the central arteries and possibly increases the risk of aortic rupture.3031 ARBs and ACEIs have a greater effect on aortic stiffness than β-blockers despite their similar effects on peripheral blood pressure.323334 Yetman, et al.16 compared the efficacy of enalapril to that of β-blockers in a non-randomized, open label study and reported that enalapril improved the aortic stiffness and distensibility in MFS patients and decreased aortic root dilatation and clinical events.
In our study, the concomitant prescription of RAAS blockade with β-blocker was negatively correlated with the risk of aortic dissection development and re-operation, especially at the aortic arch and descending thoracic aorta. The reduction in aortic growth rate by RAAS blockade was definite in the DTA and abdominal aorta at the supra-renal level, as opposed to at the IMA ostium level where there was no difference between groups. The additive effect of RAAS blockade in MFS patients who have undergone ARR might be explained by a reduction in shear stress on the central arteries through an improvement in aortic stiffness and distensibility, in addition to a molecular mechanism.
There are several limitations to this study. First of all, this was a retrospective study conducted at a single center, carrying all the limitations of this study design. Dose titration of RAAS blockade was almost entirely dependent on clinician's decision and blood pressure measurement. However, the blood pressure measurement did not strictly meet the principle to measure 3 times over 3-min interval with constant time gap since last medication, which could have influenced the outcome. Second, the mean age of group 1 patients at the time of ARR was 4.4 years younger than group 2 patients, suggesting that the natural progression of disease is more rapid in group 1 than in group 2. On univariate analysis, operation at older ages was negatively associated with major aortic events or re-operation. Although we adjusted for age in the multivariate Cox regression analysis, there is still the possibility of selection bias. Third, this study covers a relatively long time period, which necessarily includes changes in operation technique and graft prosthesis material, both of which could influence clinical results. Finally, a histologic study of re-operated patients that might show the structural or molecular effects of RAAS blockade on the aortas of MFS patients was not performed. A measurement of central pressure, augmentation index, or pulsed wave velocity that could explain this result from a hemodynamic perspective was not performed in most of patients due to it's retrospective nature.
To conclude, in MFS patients who underwent ARR, the addition of RAAS blockade to β-blocker was associated with reduction of aortic dilatation and clinical events. This result might be explained by improvement in aortic stiffness or distensibility, as well as inhibition of TGF-β signaling. Further evaluation in a prospective setting is needed to confirm the benefit.
Figures and Tables
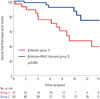 | Fig. 1Kaplan-Meier curves for cumulative survival free from major aortic events. Patients without RAAS blockade (group 1) had lower cumulative survival free of major aortic events (p=0.008). RAAS, Renin-Angiotensin-Aldosterone System. |
 | Fig. 2Mean annual rate of change in aortic diameter after aortic root replacement. Significant reduction of aortic dilatation rate by the addition of RAAS blockade was observed in descending thoracic aorta and suprarenal abdominal aorta. RAAS, Renin-Angiotensin-Aldosterone System. |
Table 1
Baseline Clinical Characteristics and Prescription Status

Table 2
Operative Information
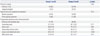
Table 3
Major Aortic Events during Follow-Up

Table 4
Univariate and Multivariate Predictors of Major Aortic Events

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A085136).
References
1. Cañadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. Part 1: pathophysiology and diagnosis. Nat Rev Cardiol. 2010; 7:256–265.

2. Shin MS, Park HY, Lim Y, Shin GJ, Jang Y, Jang BC, et al. Identification of Molecular Defects in Korean Patients with Marfan Syndrome. Korean Circ J. 2003; 33:1018–1027.

3. Jondeau G, Michel JB, Boileau C. The translational science of Marfan syndrome. Heart. 2011; 97:1206–1214.

4. Sawaki D, Suzuki T. Targeting transforming growth factor-β signaling in aortopathies in Marfan syndrome. Circ J. 2013; 77:898–899.

5. Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation. 1995; 91:728–733.
7. Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010; 47:476–485.

8. Pyeritz RE. Marfan syndrome: current and future clinical and genetic management of cardiovascular manifestations. Semin Thorac Cardiovasc Surg. 1993; 5:11–16.
9. Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC, et al. Replacement of the aortic root in patients with Marfan's syndrome. N Engl J Med. 1999; 340:1307–1313.

10. Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan's syndrome. N Engl J Med. 1994; 330:1335–1341.

11. Volguina IV, Miller DC, LeMaire SA, Palmero LC, Wang XL, Connolly HM, et al. Valve-sparing and valve-replacing techniques for aortic root replacement in patients with Marfan syndrome: analysis of early outcome. J Thorac Cardiovasc Surg. 2009; 137:1124–1132.

12. Karck M, Kallenbach K, Hagl C, Rhein C, Leyh R, Haverich A. Aortic root surgery in Marfan syndrome: Comparison of aortic valve-sparing reimplantation versus composite grafting. J Thorac Cardiovasc Surg. 2004; 127:391–398.

13. de Oliveira NC, David TE, Ivanov J, Armstrong S, Eriksson MJ, Rakowski H, et al. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2003; 125:789–796.

14. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006; 312:117–121.

15. Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med. 2008; 358:2787–2795.

16. Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am J Cardiol. 2005; 95:1125–1127.

17. Groenink M, den Hartog AW, Franken R, Radonic T, de Waard V, Timmermans J, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J. 2013; 34:3491–3500.

18. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285:1441–1446.

19. Geisbuesch S, Schray D, Bischoff MS, Lin HM, Di Luozzo G, Griepp RB. Frequency of reoperations in patients with Marfan syndrome. Ann Thorac Surg. 2012; 93:1496–1501.

20. Gott VL, Cameron DE, Alejo DE, Greene PS, Shake JG, Caparrelli DJ, et al. Aortic root replacement in 271 Marfan patients: a 24-year experience. Ann Thorac Surg. 2002; 73:438–443.
21. Nienaber CA, Von Kodolitsch Y. Therapeutic management of patients with Marfan syndrome: focus on cardiovascular involvement. Cardiol Rev. 1999; 7:332–341.
22. Ades L; CSANZ Cardiovascular Genetics Working Group. Guidelines for the diagnosis and management of Marfan syndrome. Heart Lung Circ. 2007; 16:28–30.

23. Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011; 332:361–365.

24. Nagashima H, Sakomura Y, Aoka Y, Uto K, Kameyama Ki, Ogawa M, et al. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan's syndrome. Circulation. 2001; 104:I282–I287.

25. Moltzer E, te Riet L, Swagemakers SM, van Heijningen PM, Vermeij M, van Veghel R, et al. Impaired vascular contractility and aortic wall degeneration in fibulin-4 deficient mice: effect of angiotensin II type 1 (AT1) receptor blockade. PLoS One. 2011; 6:e23411.

26. Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, et al. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012; 7:e49642.

27. Adams JN, Brooks M, Redpath TW, Smith FW, Dean J, Gray J, et al. Aortic distensibility and stiffness index measured by magnetic resonance imaging in patients with Marfan's syndrome. Br Heart J. 1995; 73:265–269.

28. Segers P, De Backer J, Devos D, Rabben SI, Gillebert TC, Van Bortel LM, et al. Aortic reflection coefficients and their association with global indexes of wave reflection in healthy controls and patients with Marfan's syndrome. Am J Physiol Heart Circ Physiol. 2006; 290:H2385–H2392.

29. Nollen GJ, Groenink M, Tijssen JG, Van Der Wall EE, Mulder BJ. Aortic stiffness and diameter predict progressive aortic dilatation in patients with Marfan syndrome. Eur Heart J. 2004; 25:1146–1152.

30. Groenink M, de Roos A, Mulder BJ, Verbeeten B Jr, Timmermans J, Zwinderman AH, et al. Biophysical properties of the normal-sized aorta in patients with Marfan syndrome: evaluation with MR flow mapping. Radiology. 2001; 219:535–540.

31. Groenink M, Langerak SE, Vanbavel E, van der Wall EE, Mulder BJ, van der Wal AC, et al. The influence of aging and aortic stiffness on permanent dilation and breaking stress of the thoracic descending aorta. Cardiovasc Res. 1999; 43:471–480.

32. Dhakam Z, McEniery CM, Yasmin , Cockcroft JR, Brown MJ, Wilkinson IB. Atenolol and eprosartan: differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006; 19:214–219.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download