To the Editor:
Hemichorea is usually associated with a unilateral lesion of the central nervous system. Causative brain lesions include stroke, neoplasm, vascular malformation, and tuberculoma.1 Drugs and systemic illness have been linked to hemichorea without brain lesions; however, such instances are extremely uncommon.2345 Levosulpiride is a highly selective dopamine D2-receptor antagonist that is widely used for the treatment of functional dyspepsia. It is reported to induce extrapyramidal syndromes, 6 although there is no description of hemichorea.
A 63-year-old, right-handed woman with a history of hyperlipidemia presented at our clinic with increasing involuntary, irregular, and purposeless movements of her lip, left arm, and leg (Supplementary Video 1, only online). Ten days prior, her daughter noticed these abnormal movements. The patient denied any family history of movement disorders. The movements were enhanced with action (i.e., walking) and disappeared during sleep. There were no fluctuations during the day. Neurological examination was normal except for the involuntary movements. Brain MRI, including diffusion-weighted imaging (DWI), was normal except for a benign-looking vascular malformation (i.e., cavernous angioma) in the left medial temporal area, and MR angiography produced normal findings (Fig. 1A and B). Her laboratory findings were unremarkable, including fasting glucose, glycated hemoglobin A, glucose tolerance, thyroid function, venereal disease research laboratory, peripheral blood smear, creatine kinase, antinuclear antibody, anticardiolipin antibody, anti-neutrophil cytoplasmic antibodies, antiphospholipid antibody, rheumatoid factor, and antistreptolysin O. Routine urinalysis and 24-hour urine collection (for creatinine clearance and protein excretion evaluation) results showed normal renal function. Somatosensory-evoked potentials for median and posterior tibial nerve stimulation were normal. The patient had been taking levosulpiride (75 mg/day), talniflumate (1110 mg/day), ranitidine (300 mg/day), afloqualone (60 mg/day), and calcium polycarbophil (1875 mg/day) for about 2 months for her arthralgia and gastrointestinal discomfort. Five days after complete medication withdrawal, the involuntary movements significantly subsided. Single photon emission computed tomography (SPECT), using 99mtechnetium-hexylmethylpropylene amine oxime (99mTc-HMPAO) at day 5, showed mild hypoperfusion in the right basal ganglia, particularly the caudate nucleus (Fig. 1C). Although her involuntary movements were improving, she continued to complain of the involuntary movements, and quetiapine (12.5 mg) was prescribed nightly. We could not increase the dosage and were forced to stop the medication after 1 week, as she complained of drowsiness. We could not determine the effect of quetiapine due to the small dose. Within 1 month, the hemichorea completely resolved. During the 11-month follow-up period, she had no recurrence of hemichorea.
Although hemichorea is not usually expected to occur in the absence of brain lesions,1 it is the most possible cause, considering the temporal relationship between levosulpiride administration and hemichorea, the rapid improvement after levosulpiride withdrawal, and normal laboratory and brain MRI findings.
The underlying mechanism of hemichorea is unknown, as various causes can lead to chorea. With the functional imaging studies of different etiologies, we can surmise that striatal dysfunction might play a role.1 Brain SPECT showed hypoperfusion in the basal ganglia, contralateral to the hemichorea in most patients with chorea associated with hyperglycemia, a finding that was similar to ours. In other cases, brain SPECT showed hyperperfusion in the basal ganglia.7 This discrepancy might be due to the time at which the SPECT was taken. Brain SPECT showing hyperperfusion in the basal ganglia during the initial study revealed hypoperfusion upon follow-up.7 Increased cerebral blood flow has been suggested to be a compensation for the rapid normalization of blood glucose level.7 Dopamine transporter imaging showed dopaminergic deficits in the basal ganglia with Huntington's disease and choreaacanthocytosis. 89 It might be difficult to draw conclusions, as these previous studies observed cases due to various causes; nevertheless, we can infer that striatal dysfunction might be a common pathophysiologic mechanism for the development of chorea. We can also postulate that our SPECT findings suggest striatal dysfunction possibly due to the D2 receptor antagonist activity of levosulpiride.
Several issues should be noted. First, the patient was taking several drugs including ranitidine, a H2 receptor antagonist, which has also been reported to induce chorea.10 However, its implication for chorea has not been explained clearly. Second, it is difficult to explain why her hemichorea occurred without brain lesions, although cases of hemichorea without brain lesions have been reported.24 Microscopic disease in the unilateral basal ganglia has been suggested to be an underlying cause.5 The hemichorea might have been caused by an acute ischemia with negative DWI; however, this was unlikely, considering the subacute onset of her hemichorea and slight symptoms at the beginning. Her vascular malformation might have contributed to the development of the hemichorea, although ipsilateral hemichorea to the extra-basal ganglia lesion is extremely rare.
Figures and Tables
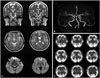 | Fig. 1Magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and single photon emission computed tomography (SPECT, using 99mTc-HMPAO) findings. (A) Routine MRI scan of the brain, including diffusion-weighted imaging (DWI), did not reveal any responsible abnormality. A nodular and signal void was noted in the left temporal anterior lobe, which was suggested to be a slow flow vascular malformation, such as cavernous angioma or venous malformation. (B) MRA showed normal results. (C) 99mTc-HMPAO SPECT showed mild hypoperfusion in the right basal ganglia, particularly in the caudate nucleus (arrow). 99mTc-HMPAO, 99mtechnetium-hexylmethylpropylene amine oxime. |
Notes
References
2. Baba M, Terada A, Hishida R, Matsunaga M, Kawabe Y, Takebe K. Persistent hemichorea associated with thyrotoxicosis. Intern Med. 1992; 31:1144–1146.

3. Dragasević N, Petković-Medved B, Svetel M, Filipović SR, Kostić VS. Paroxysmal hemiballism and idiopathic hypoparathyroidism. J Neurol. 1997; 244:389–390.

4. Lai MH, Wang TY, Chang CC, Tsai KC, Chang ST. Hemichorea associated with gabapentin therapy with hypoperfusion in contralateral basal ganglion - a case of a paraplegic patient with neuropathic pain. J Clin Pharm Ther. 2008; 33:83–86.

5. Sheen VL, Asimakopoulos F, Heyman E, Henderson G, Feske SK. Hemichorea as a presentation of recurrent non-Hodgkin's lymphoma. J Neurol. 2002; 249:1746–1748.

6. Shin HW, Kim MJ, Kim JS, Lee MC, Chung SJ. Levosulpiride-induced movement disorders. Mov Disord. 2009; 24:2249–2253.

7. Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002; 200:57–62.

8. Müller-Vahl KR, Berding G, Emrich HM, Peschel T. Chorea-acanthocytosis in monozygotic twins: clinical findings and neuropathological changes as detected by diffusion tensor imaging, FDG-PET and (123)I-beta-CIT-SPECT. J Neurol. 2007; 254:1081–1088.

SUPPLEMENTARY DATA
Video 1
To enhance the patient's hemichorea, we asked her to count backwards and walk around. The video shows the hemichorea of her left arm and leg. The video does not show her lip movements properly due to her blurred face.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download