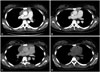
A 22-year-old woman diagnosed with Graves' disease was admitted, as an X-ray had incidentally detected mediastinal widening. A chest computed tomography (CT) scan showed a 7.6×2.0×7.7 cm [maximum cross-sectional area (MCSA) 1265.14 mm
2] thymic enlargement (
Fig. 1A). Fluorine-18 fluorodeoxyglucose (
18F-FDG) positron emission tomography (PET) results indicated that
18F-FDG uptake in the thymus was increased and heterogeneous. Therefore, the patient underwent a CT-guided needle biopsy due to our concern for possible thymoma; however, the pathologic results revealed non-neoplastic thymic tissue. The patient was treated with methimazole 15 mg/day. Her thyroid functions, measured using a radioimmunoassay kit (RIAKEY, Shin Jin Medics Inc., Goyang, Korea), were as follows: free thyroxine (T4), 1.02 ng/dL (reference range 0.70–1.80 ng/dL); total triiodothyronine (T3), 109 ng/dL (reference range 87–184 ng/dL); and thyrotropin (TSH), 0.22 µIU/mL (reference range 0.40–4.10 µIU/mL). Her serum thyrotropin binding inhibiting immunoglobulin (TBII) titer was not determined (
Table 1, visit #1). After 1 year, laboratory testing indicated poorly controlled hyperthyroidism; the patient had shown poor compliance with antithyroid medication. Serum TBII, measured as serum thyrotropin receptor (TSH-R) antibodies by using a first-generation porcine radioreceptor kit (RSR, Cardiff, UK), was 31.4% (reference range <15%). Therefore, we prescribed methimazole at an increased dose of 20 mg/day (visit #2). After 20 months at this antithyroid drug increment, the serum thyroid hormone levels and TBII were approximately normalized (
Table 1, visit #3), and the size of the thymus had decreased to 6.9×1.0×6.0 cm (MCSA 359.12 mm
2) in a follow-up CT scan (
Fig. 1B). Subsequent to visit #3, she discontinued the antithyroid medication for over 1 year against medical advice and developed thyrotoxic symptoms including sweating and weight loss. Laboratory results obtained at 54 months after the initial visit were as follows: free T4, 4.88 ng/dL (reference range 0.70–1.80 ng/dL); total T3, 282 ng/dL (reference range 87–184 ng/dL); and TSH, <0.05 µIU/mL (reference range 0.40–4.10 µIU/mL). The TBII level, measured using a second-generation recombinant human radioreceptor kit (BRAHMS, Berlin, Germany), was 14.7 IU/L (reference range 0–1.0 IU/L) (
Table 1, visit #4). The patient's antithyroid medication was restarted at a methimazole dose of 30 mg/day. Nevertheless, hyperthyroidism remained present after 9 months of therapy (
Table 1, visit #5). The size of the thymic hyperplasia increased again and measured 9.8×3.2×9.5 cm (MCSA 1759.58 mm
2) in a CT scan (
Fig. 1C). For treatment of Grave's disease refractory to the antithyroid medication, the patient underwent a total thyroidectomy after visit #5, as she preferred surgery to radioactive iodine therapy. She started to take 0.1 mg/day of levothyroxine as a thyroid hormone replacement yet took the medicine irregularly. Six months after surgery, thyroid function tests indicated that she was in a hypothyroid state, and the TBII level was not checked at that time (
Table 1, visit #6). A CT scan revealed marked shrinkage of the thymus to 6.2×1.0×6.0 cm (MCSA 305.47 mm
2) (
Fig. 1D). She was advised to take levothyroxine regularly. Eighteen months after surgery, laboratory tests revealed a free T4 level of 1.11 ng/dL (reference range 0.70–1.80 ng/dL), a total T3 level of 130 ng/dL (reference range 87–184 ng/dL), a TSH level of 6.73 µIU/mL (reference range 0.40–4.10 µIU/mL), and a decreased TBII level of 0.9 IU/L (BRAHMS, Berlin, Germany; reference range 0–1.0 IU/L) (
Table 1, visit #7).
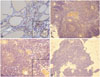
We performed an immunohistochemical study using the goat polyclonal antibodies for TSH-R (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on the patient's thyroid and thymic tissue samples and on the thymic tissue of a person without Graves' disease as a negative control (
Fig. 2). The patient's thyroid and thymic tissues showed positive TSH-R results. The follicular epithelial cells of her thyroid showed weakly positive staining results (
Fig. 2A). In her thymus, the medullary portion of the thymic lobules was predominantly stained by antibodies for TSH-R (
Fig. 2B). Furthermore, TSH-R expression was observed in Hassall's corpuscles and epithelial reticular cells rather than in lymphocytes (
Fig. 2C). In contrast, the thymic tissue of the person without Graves' disease was negative for TSH-R (
Fig. 2D).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download