Abstract
Purpose
Our previous studies have shown that oncostatin M (OSM) promotes trophoblast invasion activity through increased enzyme activity of matrix metalloproteinase (MMP)-2 and -9. We further investigated OSM-induced intracellular signaling mechanisms associated with these events in the immortalized human trophoblast cell line HTR8/SVneo.
Materials and Methods
We investigated the effects of OSM on RNA and protein expression of MMP-2 and -9 in the first-trimester extravillous trophoblast cell line (HTR8/SVneo) via Western blot. The selective signal transducer and activator of transcription (STAT)3 inhibitor, stattic, STAT3 siRNA, and extracellular signal-regulated kinase (ERK) siRNA were used to investigate STAT3 and ERK activation by OSM. The effects of STAT3 and ERK inhibitors on OSM-induced enzymatic activities of MMP-2 and -9 and invasion activity were further determined via Western blot and gelatin zymography.
Results
OSM-induced MMP-2 and -9 protein expression was significantly suppressed by STAT3 inhibition with stattic and STAT3 siRNA silencing, whereas the ERK1/2 inhibitor (U0126) and ERK silencing significantly suppressed OSM-induced MMP-2 protein expression. OSM-induced MMP-2 and MMP-9 enzymatic activities were significantly decreased by stattic pretreatment. The increased invasion activity induced by OSM was significantly suppressed by STAT3 and ERK1/2 inhibition, though to a greater extent by STAT3 inhibition.
In early pregnancy, extravillous trophoblasts (EVTs) acquire an invasive phenotype in order to profoundly infiltrate the decidua and then reach and penetrate maternal spiral arteries. The direction of invasion appears to be determined through the expression of integrins in the decidual matrix surrounding the trophoblast cell and the capacity of this cell to produce matrix metalloproteinase (MMP).1 Given that basement membranes are the major structural hindrance to invading cells, the two gelatinases MMP-2 and MMP-9 (which cleave type IV collagen, the main component of basement membranes) are considered key enzymes in the invasion process of EVTs. The invasive capacities of trophoblasts are positively and negatively regulated by numerous cytokines including leukemia inhibitory factor (LIF), interleukin (IL)-6, hepatocyte growth factor (HGF), and granulocyte macrophage-colony stimulating factor (GM-CSF).
Oncostatin M (OSM), a cytokine in the IL-6 family, is a multifunctional cytokine that affects the growth and differentiation of many different cell types. Information is lacking regarding the effects of OSM on pregnancy, although OSM concentrations in the sera of pregnant women were found to be significantly higher than those of nonpregnant women, particularly in the first trimester.2 OSM is produced at a higher level in the decidual glands and stromal cells than in chorionic tissue, and OSM receptors exist on trophoblasts and endometrial epithelial cells.2 Action of IL-6 type cytokines is attributed to the activation of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) cascades, which were induced by the activated signal transducers glycoprotein 130, LIF receptor, and OSM receptor. It has been reported that phosphorylated STAT3 is strongly associated with the invasiveness of tumors and trophoblasts, in which it is mainly activated by LIF.3
Our previous study demonstrated that OSM enhances the invasion activities of EVTs during the first trimester through increased expression of the gelatinases MMP-2 and -9.4 However, the pathways involved in OSM-mediated invasion of trophoblasts have not been investigated.
Therefore, the aim of the present study was to determine the significance of ERK- and STAT3-dependent signaling pathways in OSM-mediated invasion of trophoblasts using HTR8/SVneo cells derived from human first-trimester placenta explant cultures immortalized by SV40 large T antigen as a model of first-trimester trophoblasts.5
The EVT cell line HTR8/SVneo was kindly provided by Dr. Charles Graham (Queen's University, Kingston, ON, Canada). This cell line was produced via the immortalization of HTR8 cells, an EVT cell line from primary explant cultures of first-trimester human placenta (8 to 10 weeks of gestation), with SV40.678 In the present study, HTR8/SVneo cells were used between passages 70 and 75.
HTR8/SVneo cells were cultured in RPMI-1640 (GIBCO, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS). To analyze the effects of OSM on MMP-2 and MMP-9 in HTR8/SVneo cells, 1×107 cells were seeded in a 100-mm culture dish. After 24 or 48 h, the cells were treated with recombinant human OSM (20 ng/mL; Sigma-Aldrich, St. Louis, MO, USA).
HTR8/SVneo cells were cultured to 70–80% confluency and incubated for 48 h, with or without OSM (20 ng/mL). Western blot was performed as described previously.4 We checked active forms of MMP-2 (63 kDa) and MMP-9 (92 kDa).
The activation of STAT3 by OSM was confirmed in a previous study.9 The cells were treated with OSM (20 ng/mL) for 48 h with or without pretreatment with the STAT3 inhibitor, stattic (Tocris, R&D system, Minneapolis, MN, USA), at 0.5 µM or 1 µM for 1 h in dimethyl sulfoxide (DMSO) and subjected to Western blotting as described above.
The cells were treated with OSM (20 ng/mL) for 5 min, 15 min, 30 min, 1 h, 3 h, or 8 h. Control cells were incubated for 8 h without OSM. Western blotting was performed as described above with the following antibodies: mouse anti-human phosphorylated ERK1/2 (phosphorylation of Thr202/Tyr704; Cat no. #9106S, 1:2000) and mouse anti-human total ERK1/2 (Cat no. #4696S, 1:2000; Cell Signaling Technology, Danvers, MA, USA).
The cells were treated with OSM (20 ng/mL) for 48 h with or without pretreatment with the ERK inhibitor U0126 [10 µM or 25 µM for 1 h in phosphate buffered saline (PBS)] and analyzed via Western blotting as described above.
The double-stranded siRNA oligonucleotide against STAT3 had the sequence 5'-AATGTTCTCTATCAGCACAAT-3'.10 Oligonucleotides were synthesized by Genolution Pharmaceuticals, Inc. (Seoul, Korea). Negative controls consisted of a well-tested non-targeting scrambled siRNA with no homology to mammalian genes. Control siRNA (no. 6568) and ERK1/2 siRNA (no. 6560) were purchased from Cell Signaling Technology, Inc. HTR-8/SVneo cells were seeded in 6-well plates immediately before transfection. For optimum transfection ef-ficacy, cells were seeded to a final confluency of 30–50%. Cells were transfected with either STAT3 siRNA (25 nM) or scrambled siRNA (25 nM) complexed with G-Fectin (Genolution Pharmaceuticals, Inc., Seoul, Korea) for 24 h. The other cells were transfected with either control siRNA (100 nM) or ERK siRNA (100 nM) complexed with G-Fectin for 24 h. After treatment with OSM (20 ng/mL) for 48 h, cells were detached and analyzed via Western blotting.
A gelatin zymography assay was performed as described previously.4 The cells were treated with OSM (20 ng/mL) for 48 h with or without pretreatment with the STAT3 inhibitor stattic at 0.5, 1, or 2.5 µM for 1 h.
A Matrigel invasion assay was performed as described previously.4 The invasive ability of HTR8/SVneo cells was examined using a cell invasion assay kit with a polycarbonate membrane (pore size: 8 µm; CHEMICON, Billerica, MA, USA) on ECMatrixTM. Cells were prepared at a concentration of 1×106 cells/mL. For the invasion assay, 3×105 cells suspended in 300 µL of serum-free RPMI medium were added to the upper chamber of the membrane, and 500 µL of RPMI medium containing 10% FBS with or without stattic (1 µM) or U0126 (25 µM) was added to the lower chamber. After 1 h, OSM (20 ng/mL) was added to the lower chamber. The plates were incubated at 37℃ under 5% CO2 and 95% air for 48 h. At the end of the incubation, the cells on the upper filter surface were completely removed by wiping with a cotton swab. Invasive cells on the lower surface of the membrane were stained by immersing the inserts in the staining solution for 20 min, rinsed several times by dipping the inserts in a beaker of water, and then air-dried. The number of cells that passed through the membrane and migrated to the other side was quantitated by dissolving stained cells in 10% acetic acid and transferring a dye-solute mixture to a 96-well plate in order to obtain a spectrophotometer reading of optical density (OD) at 560 nm.
Each experiment was performed three times, and all data are expressed as mean±SEM. The non-parametric Mann-Whitney rank-sum test and an independent t-test were used to compare the two groups. A p-value<0.05 was considered to be statistically significant. Data were analyzed with SPSS software (SPSS Inc., Chicago, IL, USA).
To investigate the role of the STAT3 pathway in the OSM-induced upregulation of MMP-2 and MMP-9, HTR8/SVneo cells were pretreated with stattic (0.5, 1, or 2.5 µM) and then stimulated with OSM (20 ng/mL, 48 h). Based on Western blotting, the increased expression of MMP-2 and MMP-9 induced by OSM was significantly decreased by stattic pretreatment regardless of the concentration used (all p<0.05) (Fig. 1A and B). Transfection of HTR8/SVneo cells with 25 nM STAT3-specific siRNA (and not scrambled siRNA) significantly decreased the amount of STAT3 and phosphorylated STAT3, as shown in the previous study.9 Transfection of HTR8/SVneo cells with STAT3 siRNA also significantly decreased the expression of MMP-2 and MMP-9 that was increased by OSM (p<0.05 for both), without affecting expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein (Fig. 1C and D). Non-targeting negative control siRNA did not affect the expression of MMP-2 and MMP-9.
Basal levels of ERK phosphorylation were very low, although stimulation with OSM led to immediate and transient increases in phosphorylation (Fig. 2A). Total ERK protein expression was not significantly changed at any time point. OSM-induced ERK phosphorylation was significantly suppressed by treatment with the ERK inhibitor U0126 (25 µM for 1 h) (Fig. 2B and C).
To investigate the role of the ERK pathway in OSM-induced upregulation of MMP-2 and MMP-9, HTR8/SVneo cells were pretreated with U0126 (10 or 25 µM for 1 h) and then stimulated with OSM (20 ng/mL, 48 h). Western blot analysis showed that the induction of MMP-2 by OSM was significantly decreased by U0126 pretreatment regardless of the concentration used (p<0.05) (Fig. 3A and B). However, U0126 pretreatment did not affect the induction of MMP-9 by OSM.
Transfection of HTR8/SVneo cells with 25 nM of ERK-specific siRNA (and not scrambled oligonucleotide) significantly decreased the cellular content of total ERK (30.5%; p<0.05) and phosphorylated ERK (52.0%; p<0.05) (Fig. 3C and D). After transfection of HTR8/SVneo cells with ERK siRNA, protein expression of MMP-2 and MMP-9 induced by OSM was significantly lower than OSM-induced expression of MMP-2 and -9 without transfection (p<0.05 for both). Non-targeting negative-control siRNA did not significantly affect the expression of MMP-2 and MMP-9.
On the gelatinolytic zymography, OSM-induced MMP-2 and MMP-9 enzymatic activities were significantly decreased by stattic pretreatment, regardless of the concentration used (all p<0.05) (Fig. 4A and B). In the ECMatrix invasion assay, OSM significantly increased the invasion activities of HTR8/SVneo cells (Fig. 4C). Pretreatment with stattic or U0126 before OSM treatment decreased the number of invaded cells more than with OSM treatment alone, and this effect was more pronounced with stattic pretreatment (p<0.01 and p<0.05, respectively) (Fig. 4C and D).
MMP-2 and MMP-9 are important enzymes in the invasion of trophoblasts during early pregnancy.1 We previously reported that OSM induced the expression of both enzymes in the HTR8/SVneo cells.4 Using specific pharmacologic inhibitors and siRNAs, we were able to demonstrate that major pathways of the IL-6 family, including ERK and STAT3 pathways, mediate the induction of these proteases via OSM. The STAT3 pathway appears to be critical for the OSM-mediated increase in the invasive activity and the number of HTR8/SVneo cells through both MMP-2 and MMP-9, whereas the ERK inhibitor did not affect OSM-induced MMP9 expression, and the effects of ERK inhibitor were weaker than those of STAT3 inhibitor in the invasion assay. Disruption of OSM signaling may be a strategy for controlling the invasiveness of trophoblasts during early pregnancy.
Dysregulation of STAT3 activity patterns during placental development has been suggested as a mechanism of pathologic processes in diseases related to defective placentation, hypoinvasion in preeclampsia, and neoplasms such as human choriocarcinoma.1112 Several soluble factors that are generally present in the decidua, mainly HGF, GM-CSF, IL-6, IL-11, and LIF, have been shown to use JAK/STAT pathways for signaling and may thereby influence invasiveness; in particular, phosphorylated STAT3 enhances the invasiveness of trophoblast cells.31213 Although other pathways, including STAT1 and STAT5, need to be investigated, our results suggest that OSM is involved in the invasion process in HTR8/SVneo cells through the STAT3 pathway. Studies have explored the functions of OSM in cancer, bone metabolism, liver regeneration, and conditions involving chronic inflammation including rheumatoid arthritis, lung and skin inflammatory disease, atherosclerosis, and cardiovascular disease. OSM stimulation of human and canine osteosarcoma cells induce STAT3 activation, thereby enhancing the expression/activation of MMP2 and VEGF, and ultimately promoting invasive behavior and tumor angiogenesis.14 OSM induced fascin through a STAT3 activation and JAK/STAT signaling pathway contributed to a migratory and invasive phenotype of breast cancer cells, in both mouse and human systems.15 For physiologic and pathologic relevance, it might be more important to study the responses of human primary trophoblasts; however, it has been difficult to study the role of these factors in the regulation of EVT invasion in vitro due to technical difficulties in obtaining a pure population of EVTs in primary cultures. Invasion of trophoblasts is regulated by numerous components, such as cytokines, endocrine systems, immune systems, and environmental factors including oxygen, and achieved by modulation of protease activity, altered cell adhesion, and induced apoptosis.16 In particular, a hypoxic condition of low oxygen partial pressure is the extrinsic factor that typically regulates the invasive ability of trophoblasts. It has been reported that the environment during EVT invasion process of the early pregnancy shows low oxygen (2–5% O2) or low glucose concentrations (1 mM).1718 The studies about invasion of trophoblast have been performed using a various models of cell line including choriocarcinoma (BeWo, JAR, and JEG3)1920 and SV40-transfection (SGHPL-4 and HTR-8/SVneo).47212223 Given the diversity of mRNA expression patterns among trophoblast cell lines, it has been suggested that the verification of the critical steps in molecular studies in trophoblast cell lines a is necessarily in an appropriate primary model system.24 Additionally, it has been reported that different cell lines display different responses to culture in 3% oxygen with respect to apoptosis, proliferation, and secreted proteases.25 Therefore, our next step might be to perform experiments under a hypoxic environment, using cell lines and primary trophoblasts.
We previously reported that OSM stimulates the migration and proliferation of HTR8/SVneo cells with downregulation of E-cadherin and is related to the STAT3 pathway.9 We cannot discriminate whether cell invasion is increased due to increased invasiveness or proliferation. However, both were significantly decreased by STAT3 inhibitor. It has been suggested that mammalian target of rapamycin (mTOR) signaling is a major mechanism within a tightly regulated network of intracellular signal pathways including the JAK/STAT system that regulate invasion in human trophoblasts by secretion of enzymes that remodel the extracellular matrix, such as MMP-2, MMP-9, uPA, and PAI-1.8 Therefore, future studies are needed to investigate the relationship between OSM and mTOR signaling.
In conclusion, this study demonstrated that both STAT3 and ERK signaling pathways are related to OSM-induced invasion activity of trophoblasts via upregulation of the expression of MMP-2 and MMP-9. The activation STAT3 appears to be critical. To better understand STAT-mediated trophoblast invasion induced by OSM, substantial research efforts should be directed toward understanding the regulation of STAT-responsive genes and their physiologic relevance during these processes in primary trophoblasts, under different conditions.
Figures and Tables
 | Fig. 1Effects of OSM, STAT3 inhibitor, and STAT3 siRNA on protein expression of MMP-2 and MMP-9. Cells were pretreated with the STAT3 inhibitor stattic (0.5, 1.0, and 2.5 µM) for 1 h before treatment with or without OSM (20 ng/mL). Protein expression of MMP-2 and MMP-9 was analyzed via Western blotting (A). The expression was quantified and expressed as mean±SEM (B). Western blot analysis of MMP-2 and MMP-9 24 h after siRNA transfection with or without OSM treatment for 48 h (C). OSM-induced protein expression of MMP-2 and MMP-9 was significantly decreased after transfection with STAT3 siRNA compared to expression without siRNA, whereas GAPDH protein expression was not affected. Non-targeting negative control siRNA did not affect OSM-induced MMP-2 and -9 protein expression. The results are representative of three independent experiments. The expression was quantified and expressed as mean±SEM (D). *p<0.05. OSM, oncostatin M; MMP, matrix metalloproteinase; STAT, signal transducer and activator of transcription; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
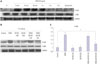 | Fig. 2Effects of OSM and ERK inhibitor (U0126) on protein expression of total and phosphorylated ERK. ERK phosphorylation in HTR8/SVneo cells was transiently stimulated by OSM (A). The effect of OSM on ERK phosphorylation was inhibited by pretreatment with U0126 (10 or 25 µM for 1 h) compared to OSM treatment alone (B). The expression was quantified and expressed as mean±SEM (C) compared to the control, *p<0.05. OSM, oncostatin M; ERK, extracellular signal-regulated kinase. |
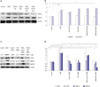 | Fig. 3Effects of OSM, ERK inhibitor (U0126), and ERK siRNA on the protein expression of MMP-2 and MMP-9. Cells were pretreated with the ERK inhibitor U0126 (10 and 25 µM) for 1 h before treatment with or without OSM (20 ng/mL) for 48 h. Protein expression of MMP-2 and MMP-9 was analyzed via Western blotting (A). The expression was quantified and expressed as mean±SEM (B). U0126 significantly suppressed OSM-induced MMP-2 expression (p<0.05) yet did not affect OSM-induced MMP-9 expression. Western blot analysis of MMP-2 and MMP-9 expression 24 h after transfection with ERK siRNA with or without OSM treatment for 48 h (C). OSM-induced expression of MMP-2 and MMP-9 was significantly decreased by ERK siRNA compared to OSM alone, with no effect on GAPDH protein expression. Non-targeting negative control siRNA did not affect OSM-induced MMP-2 and -9 protein expression. The results are representative of three independent experiments. The expression was quantified and expressed as mean±SEM (D). Data indicate mean±SEM; *p<0.05. OSM, oncostatin M; ERK, extracellular signal-regulated kinase; MMP, matrix metalloproteinase. |
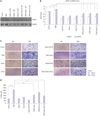 | Fig. 4Effects of STAT3 inhibitor on OSM-induced MMP-2 and MMP-9 enzymatic activities and invasion activity. On the gelatinolytic zymography, OSM-induced MMP-2 and MMP-9 enzymatic activities were significantly decreased by stattic pretreatment (0.5, 1.0, and 2.5 µM) for 1 h, regardless of the concentration used (A). Numerical data were evaluated statistically and are presented in a histogram (B). ECMatrix invasion by HTR8/SVneo cells after treatment with OSM (20 ng/mL) for 48 h, with or without STAT3 or ERK inhibitors (stattic and U0126 respectively). (C) Invading cells were analyzed using a trans-insert system. The cells were stained with hematoxylin and eosin for quantification of the invading cells (40×, scale bar=100 µm and 200×, scale bar=20 µm). (D) Columns in the graphs show the number of invading HTR8/SVneo cells. Data are expressed as mean±SEM. Significant differences are indicated (*p<0.05, **p<0.01). OSM, oncostatin M; ERK, extracellular signal-regulated kinase; MMP, matrix metalloproteinase; STAT, signal transducer and activator of transcription. |
ACKNOWLEDGEMENTS
We thank Dr. Charles H. Graham (Department of Anatomy and Cell Biology, Queen's University, Kingston, Ontario, Canada) for generously supplying the HTR8/SVneo cells. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2014R1A1A1004279), and Seoul St. Mary's Hospital Clinical Medicine Research Program (2013) through the Catholic University of Korea. The English in this document has been checked and edited by eWorldEditing, Inc.
References
1. Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006; 27:783–793.

2. Ogata I, Shimoya K, Moriyama A, Shiki Y, Matsumura Y, Yamanaka K, et al. Oncostatin M is produced during pregnancy by decidual cells and stimulates the release of HCG. Mol Hum Reprod. 2000; 6:750–757.

3. Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, et al. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol. 2005; 37:2284–2296.

4. Ko HS, Kang HK, Kim HS, Choi SK, Park IY, Shin JC. The effects of oncostatin M on trophoblast cells: influence on matrix metalloproteinases-2 and -9, and invasion activity. Placenta. 2012; 33:908–913.

5. Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993; 206:204–211.

6. Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VK, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995; 16:413–433.

7. Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000; 62:739–747.

8. Busch S, Renaud SJ, Schleussner E, Graham CH, Markert UR. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp Cell Res. 2009; 315:1724–1733.

9. Ko HS, Choi SK, Kang HK, Kim HS, Jeon JH, Park IY, et al. Oncostatin M stimulates cell migration and proliferation by down-regulating E-cadherin in HTR8/SVneo cell line through STAT3 activation. Reprod Biol Endocrinol. 2013; 11:93.

10. Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005; 26:Suppl A. S37–S41.

11. Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002; 8:945–954.
12. Suman P, Malhotra SS, Gupta SK. LIF-STAT signaling and trophoblast biology. JAKSTAT. 2013; 2:e25155.

13. Fitzgerald JS, Poehlmann TG, Schleussner E, Markert UR. Trophoblast invasion: the role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum Reprod Update. 2008; 14:335–344.

14. Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011; 11:125.

15. Snyder M, Huang XY, Zhang JJ. Signal transducers and activators of transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J Biol Chem. 2011; 286:38886–38893.

16. Pijnenborg R. Implantation and immunology: maternal inflammatory and immune cellular responses to implantation and trophoblast invasion. Reprod Biomed Online. 2002; 4:Suppl 3. 14–17.

17. Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997; 277:1669–1672.

18. De Marco CS, Caniggia I. Mechanisms of oxygen sensing in human trophoblast cells. Placenta. 2002; 23:Suppl A. S58–S68.

19. Zygmunt M, Hahn D, Münstedt K, Bischof P, Lang U. Invasion of cytotrophoblastic JEG-3 cells is stimulated by hCG in vitro. Placenta. 1998; 19:587–593.

20. Mandl M, Haas J, Bischof P, Nöhammer G, Desoye G. Serum-dependent effects of IGF-I and insulin on proliferation and invasion of human first trimester trophoblast cell models. Histochem Cell Biol. 2002; 117:391–399.

21. Liu HY, Jia XQ, Gao LX, Ma YY. Hepatocyte growth factor regulates HLX1 gene expression to modulate HTR-8/SVneo trophoblast cells. Reprod Biol Endocrinol. 2012; 10:83.

22. Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol. 1999; 128:181–189.

23. Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999; 20:661–667.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download