Abstract
Purpose
The pathophysiology of discogenic low back pain is not fully understood. Tetrodotoxin-sensitive voltage-gated sodium (NaV) channels are associated with primary sensory nerve transmission, and the NaV1.7 channel has emerged as an analgesic target. Previously, we found increased NaV1.7 expression in dorsal root ganglion (DRG) neurons innervating injured discs. This study aimed to examine the effect of blocking NaV1.7 on sensory nerves after disc injury.
Materials and Methods
Rat DRG neurons innervating the L5/6 disc were labeled with Fluoro-Gold (FG) neurotracer. Twenty-four rats underwent intervertebral disc puncture (puncture group) and 12 rats underwent sham surgery (non-puncture group). The injury group was divided into a saline infusion group (puncture+saline group) and a NaV1.7 inhibition group, injected with anti-NaV1.7 antibody (puncture+anti-NaV1.7 group); n=12 per group. Seven and 14 days post-surgery, L1 to L6 DRGs were harvested and immunostained for calcitonin gene-related peptide (CGRP) (an inflammatory pain marker), and the proportion of CGRP-immunoreactive (IR) DRG neurons of all FG-positive neurons was evaluated.
Results
The ratio of CGRP-IR DRG neurons to total FG-labeled neurons in the puncture+saline group significantly increased at 7 and 14 days, compared with the non-puncture group, respectively (p<0.05). Application of anti-NaV1.7 into the disc significantly decreased the ratio of CGRP-IR DRG neurons to total FG-labeled neurons after disc puncture at 7 and 14 days (40% and 37%, respectively; p<0.05).
Human intervertebral disc degeneration is thought to be a source of back pain; however, the patho-mechanism is not fully understood. Pain mechanisms have been explored using animal disc degeneration models, samples harvested from painful human discs, MRI studies, and biomechanical studies. In two review articles, Ohtori, et al.1 and Lotz and Ulrich2 reported that painful discs are characterized by a confluence of innervation, inflammation, and mechanical hypermobility.
In several human and animal studies, sensory nerve fibers in degenerated discs were shown to express painful neuropeptides and growth factors, such as substance P (SP)34 and calcitonin gene-related peptide (CGRP)56 as well as nerve growth factors.7 Furthermore, it has been reported in animal models that such neuropeptides are up-regulated in dorsal root ganglion (DRG) neurons innervating intervertebral discs after disc injury or during inflammation and degeneration. Thus, these peptides may be a target for treatment of discogenic pain.89
Voltage-gated sodium (NaV) channels are a class of transmembrane proteins that conduct current and enable fast cellular depolarization.10 Nine functionally unique mammalian NaV alpha subunits (NaV1.1–1.9) have been identified and cloned.11 Painful genetic disorders, such as primary erythromelalgia and paroxysmal extreme pain disorder,1213 occur when the SNC9A gene encoding the alpha subunit of NaV1.7 is mutated to alter channel activity. In contrast, truncation of the gene or loss-of-function mutations can result in conditions in which individuals are unable to feel pain.14 Thus, of the nine NaV subunits, NaV1.7 represents the most promising analgesic target to date. Interestingly, it was reported that the SNC9A gene is closely associated with knee osteoarthritis (OA) pain, and an amino acid change in the NaV1.7 α-chain is associated with knee pain in patients with OA.1516 Previously, we evaluated pain-related expression of NaV1.7 in DRG neurons innervating punctured intervertebral discs in a rat animal model.17 Disc injury was shown to increase NaV1.7 expression in DRG neurons.17 This suggested that NaV1.7 may be a therapeutic target for pain in patients with disc degeneration.
The purpose of the current study was to examine the effect of blocking NaV1.7 on sensory nerves after disc injury in rats.
All protocols for animal procedures were approved by the Ethics Committees of Chiba University in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision).
Thirty-six male Sprague-Dawley rats weighing 220–250 g were used. Animals were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). All animals underwent a midline ventral longitudinal incision to expose the L5/6 intervertebral disc. Approximately 10 µg of Fluoro-Gold neurotracer crystals (FG; Fluorochrome, Denver, CO, USA) were applied to the surface of the L5/6 intervertebral disc to label the DRG neurons innervating the discs. Ten minutes after FG application, 24 rats underwent intervertebral disc puncture, in which each disc was punctured five times with a 23-gauge needle (puncture group), and 12 rats were used as non-puncture controls (non-puncture group). The puncture group was divided into a 10 µL saline infusion group (puncture+saline group) and a NaV1.7 inhibition group (puncture+anti-NaV1.7 group), injected with 2.5 µg of anti-NaV1.7 antibody (10 µL; Alomone Labs Ltd., Jerusalem, Israel); n=12 animals per group. The hole was immediately sealed with cyanoacrylate adhesive to prevent leakage of anti-NaV1.7 antibody or saline, and the skin was closed. The puncture procedure was performed according to our previously reported methods.18
CGRP immunostaining was evaluated 7 and 14 days post-injury (7 days: non-puncture group, n=6; puncture+saline group, n=6; and puncture+anti-NaV1.7 group, n=6; 14 days: non-puncture group, n=6; puncture+saline group, n=6; and puncture+anti-NaV1.7 group, n=6). Rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and transcardially perfused with 500 mL of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). From a dorsal approach, the back muscles and lamina were removed, and bilateral DRGs from levels L1 to L6 were resected. The specimens were immersed in the same fixative solution overnight at 4℃. After incubation in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 20 hours at 4℃, each ganglion was sectioned at a thickness of 10 µm on a cryostat and mounted on Poly-L-Lysine-coated slides.
Sections were blocked in a solution consisting of 0.01 M PBS containing 0.3% Triton X-100 and 3% skim milk for 60 min. Sections were then incubated with rabbit anti-CGRP (Chemicon, Temecula, CA, USA), diluted 1:1000 in blocking solution, for 20 hours at 4℃, followed by incubation with goat anti-rabbit Alexa-488 fluorescent antibody conjugate for visualization (1:400; Molecular Probes, Eugene, OR, USA). After each step, the sections were rinsed three times in 0.01 M PBS.
Ten sections were selected at random for evaluation. The sections were examined using a fluorescence microscope (Nikon, Tokyo, Japan). The number of FG-labeled neurons and FG-labeled/CGRP-immunoreactive (IR) neurons was counted. The number of IR-DRG neurons per 0.45 mm2 was counted at 400×magnification using a counting grid.
The average ratio of FG-labeled/CGRP-IR neurons in each DRG was compared between groups using Welch's unpaired t test. The distribution of FG-labeled DRG neurons and CGRP-IR DRG neurons was analyzed using non-repeated measures ANOVA with the Bonferroni post hoc correction. p<0.05 was considered statistically significant. Results are reported as the mean±SEM.
FG-labeled DRG neurons innervating the L5/6 disc were present in bilateral DRGs from levels L1 through L6 (Table 1, Fig. 1). There were significantly more FG-labeled DRG neurons at the L2 level than at any other level (p<0.05) (Table 1). There was no significant difference in distribution of FG-labeled neurons between right and left sides (p>0.05). There was no significant difference in FG-labeled neurons among the three groups (non-puncture group, puncture+saline group, and puncture+anti-NaV1.7 group) on either day 7 or day 14 (p>0.05) (Table 1).
Fig. 1 shows FG-labeled and FG-labeled/CGRP-IR neurons from the three groups. Figs. 2 and 3 show the ratio of FG-labeled/CGRP-IR neurons to FG-labeled neurons at each level among the three groups. The ratio of CGRP-IR DRG neurons to total FG-labeled neurons from L1 to L6 in the puncture+saline group significantly increased at 7 and 14 days post-surgery (56±11% and 53±10%, respectively; mean±SE), compared with the non-puncture group at 7 and 14 days (34±6% and 33±7%, respectively; p<0.05). The ratio of CGRP-IR DRG neurons to total FG-labeled neurons from L1 to L6 in the puncture+saline group was significantly higher at 7 and 14 days, compared with the puncture+NaV1.7 antibody group (40±9% and 37±8%, respectively; p<0.05). The ratio of CGRP-IR DRG neurons to total FG-labeled neurons at L5 and L6 in the puncture+NaV1.7 antibody group was significantly greater at 7 days than in the non-puncture group (p<0.05). However, at 14 days, there was no significant difference in the ratio between the puncture+NaV1.7 antibody group and non-puncture group (p>0.05).
In the current study, we showed that CGRP expression increases in disc-innervating DRG neurons after disc puncture. However, NaV1.7 antibody suppressed CGRP expression in disc DRG neurons.
Recently, it was reported that the ventral and dorsal portions of the rat L5/6 disc are multisegmentally innervated by the T13 to L2 DRGs via paravertebral sympathetic trunks. These discs are also directly innervated by the L3 to L6 DRGs through the sinuvertebral nerves on the posterior longitudinal ligament.118 Furthermore, the ventral and dorsal portions of the rat L5/6 disc are mainly innervated by DRG neurons at the L2 level. In the current study, a similar pattern of innervation was observed. For patients with lumbar discogenic pain, blocking the spinal nerves at L5/6 can be effective, although for some patients it is more effective to block the L2 spinal nerves or paravertebral sympathetic trunks.1 In rats, the dorsal portion of the L5/6 disc has been shown to be dually innervated; if this pattern also occurs in humans, this could account for the different clinical outcomes.
Several cytokines, such as tumor necrosis factor and interleukins, as well as growth factors, such as NGF, have been reported to be key factors associated with discogenic low back pain in humans. The use of inhibitors or antibodies against TNF alpha, IL-6, and NGF has been evaluated in clinical trials for back pain.1278 Among these, anti-NGF therapy has shown the highest efficacy for back pain in patients.1920 It is thought that the efficacy of anti-NGF is due to the relationship between NGF and CGRP, an inflammatory neuropeptide found in small DRG neurons related to pain transmission. Half of the small DRG neurons related to pain perception are sub-classified into NGF-dependent neurons,2122 and NGF-dependent neurons contain neuropeptides, such as SP and CGRP.2122 Some reports have indicated that the proportion of NGF-independent neurons innervating the skin is higher than that of NGF-dependent neurons.2324 The proportions of NGF-independent and NGF-dependent neurons expressing CGRP that innervate the rat lumbar disc have been reported at 0.6% and 44%, respectively.25 In humans, CGRP-IR nerve fibers are present in lumbar intervertebral discs, while NGF-independent nerve fibers are not.26 Based on these reports, it is likely that the NGF and CGRP pathways could play an important role in pain transmission from the intervertebral discs. In the current study, we used CGRP as a pain marker, and found increased CGRP in DRG neurons innervating punctured discs, suggesting that it may be related to disc pain in humans.
Several authors have reported a relationship between NaV1.7 and pain in both humans and animal models. NaV1.7 expression was shown to increase in the peripheral nerves and DRG neurons after tissue and joint inflammation or peripheral nerve injury.272829 Furthermore, some authors have suggested that inhibition of NaV1.7 activity is a therapeutic target for several types of pain. It has been reported that inflammatory hyperalgesia is reduced when NaV1.7 is knocked down in mouse primary afferent neurons.3031 Moreover, DRG expression of NaV1.7 was involved in injury-induced neuronal hyperexcitability in a burn-injury model, and blocking NaV1.7 attenuated the hyperexcitability.32 It was suggested that NaV1.7 inhibition may be an effective tool for pain control in patients. In an animal disc injury model, we previously evaluated pain-related expression of NaV1.7 in DRG neurons innervating punctured intervertebral discs in rats.17 In the current study, increased expression of CGRP was observed in the DRG neurons after disc injury, and NaV1.7 antibody suppressed the expression of CGRP. These results strongly suggest that blocking NaV1.7 may be an effective therapeutic strategy for pain treatment.
Our study has several limitations that should be noted. First, we did not examine other cytokines or neuropeptides in DRG neurons innervating the discs. Second, we did not directly evaluate pain behavior, because it is very difficult to evaluate low back pain in animal models. Further studies are thus needed to more directly examine the role of NaV1.7 in discogenic low back pain.
In conclusion, CGRP expression in disc-innervating DRG neurons increased after disc puncture. However, NaV1.7 antibody suppressed CGRP expression in disc DRG neurons. Anti-NaV1.7 antibody is a potential therapeutic target for pain control in patients with lumbar disc degeneration.
Figures and Tables
Fig. 1
FG-labeled (A, B, and C) and CGRP-IR (D, E, and F) DRG neurons among 3 group. FG-labeled (A) and CGRP-IR (D) DRG neurons in the non-puncture group. FG-labeled (B) and CGRP-IR (E) DRG neurons in the puncture+saline group. FG-labeled (C) and CGRP-IR (F) DRG neurons in the puncture+anti-NaV1.7 antibody group. Arrowheads indicate FG-labeled/CGRP-IR DRG neurons. Magnification rate is ×400. FG, Fluoro-Gold; CGRP-IR, calcitonin gene-related peptide-immunoreactive; DRG, dorsal root ganglion; NaV, voltage-gated sodium.
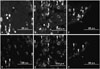
Fig. 2
Ratio of FG-labeled/CGRP-IR DRG neurons to FG-labeled DRG neurons in the non-puncture, puncture+saline, and puncture+anti-NaV1.7 antibody groups on day 7. The ratio at levels L1 to L6 in the puncture+saline group was significantly greater than the non-puncture and the puncture+NaV1.7 antibody groups (*p<0.05). The ratio at levels L5 and L6 in the puncture+NaV1.7 antibody group was significantly greater than the non-puncture group (†p<0.05). FG, Fluoro-Gold; CGRP-IR, calcitonin gene-related peptide-immunoreactive; DRG, dorsal root ganglion; NaV, voltage-gated sodium.
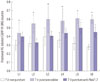
Fig. 3
Ratio of FG-labeled/CGRP-IR DRG neurons to FG-labeled DRG neurons in the non-puncture, puncture+saline, and puncture+anti-NaV1.7 antibody groups on day 14. The ratio at levels L1 to L6 in the puncture+saline group was significantly greater than in the non-puncture and the puncture+NaV1.7 antibody groups (*p<0.05). The ratio in the puncture+NaV1.7 antibody group was not significantly different than the non-puncture group (p>0.05). FG, Fluoro-Gold; CGRP-IR, calcitonin gene-related peptide-immunoreactive; DRG, dorsal root ganglion; NaV, voltage-gated sodium.
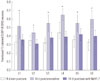
Table 1
Total Number of FG-Labeled Neurons at Each Level

References
1. Ohtori S, Inoue G, Miyagi M, Takahashi K. Pathomechanisms of discogenic low back pain in humans and animal models. Spine J. 2015; 15:1347–1355.

2. Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006; 88:Suppl 2. 76–82.

3. Ashton IK, Walsh DA, Polak JM, Eisenstein SM. Substance P in intervertebral discs. Binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994; 65:635–639.

4. Coppes MH, Marani E, Thomeer RT, Groen GJ. Innervation of "painful" lumbar discs. Spine (Phila Pa 1976). 1997; 22:2342–2349.

5. Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine (Phila Pa 1976). 1995; 20:2645–2651.
6. McCarthy PW, Carruthers B, Martin D, Petts P. Immunohistochemical demonstration of sensory nerve fibers and endings in lumbar intervertebral discs of the rat. Spine (Phila Pa 1976). 1991; 16:653–655.

7. Miyagi M, Ishikawa T, Orita S, Eguchi Y, Kamoda H, Arai G, et al. Disk injury in rats produces persistent increases in pain-related neuropeptides in dorsal root ganglia and spinal cord glia but only transient increases in inflammatory mediators: pathomechanism of chronic diskogenic low back pain. Spine (Phila Pa 1976). 2011; 36:2260–2266.

8. Aoki Y, Ohtori S, Ino H, Douya H, Ozawa T, Saito T, et al. Disc inflammation potentially promotes axonal regeneration of dorsal root ganglion neurons innervating lumbar intervertebral disc in rats. Spine (Phila Pa 1976). 2004; 29:2621–2626.

9. Hayashi Y, Ohtori S, Yamashita M, Yamauchi K, Inoue G, Suzuki M, et al. Direct single injection of p38 mitogen-activated protein kinase inhibitor does not affect calcitonin gene-related peptide expression in dorsal root ganglion neurons innervating punctured discs in rats. Spine (Phila Pa 1976). 2009; 34:2843–2847.

10. Rupasinghe DB, Knapp O, Blomster LV, Schmid AB, Adams DJ, King GF, et al. Localization of Nav 1.7 in the normal and injured rodent olfactory system indicates a critical role in olfaction, pheromone sensing and immune function. Channels (Austin). 2012; 6:103–110.

11. King GF, Escoubas P, Nicholson GM. Peptide toxins that selectively target insect Na(V) and Ca(V) channels. Channels (Austin). 2008; 2:100–116.
12. Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005; 11:555–562.

13. Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006; 52:767–774.

14. Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006; 444:894–898.

15. Reimann F, Cox JJ, Belfer I, Diatchenko L, Zaykin DV, McHale DP, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci U S A. 2010; 107:5148–5153.
16. Valdes AM, Arden NK, Vaughn FL, Doherty SA, Leaverton PE, Zhang W, et al. Role of the Nav1.7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken). 2011; 63:440–444.

17. Sadamasu A, Sakuma Y, Suzuki M, Orita S, Yamauchi K, Inoue G, et al. Upregulation of NaV1.7 in dorsal root ganglia after intervertebral disc injury in rats. Spine (Phila Pa 1976). 2014; 39:E421–E426.

18. Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine (Phila Pa 1976). 2001; 26:946–950.

19. Gimbel JS, Kivitz AJ, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain. 2014; 155:1793–1801.

20. Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain. 2013; 154:1009–1021.

21. Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995; 7:1484–1494.

22. Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998; 20:629–632.

23. Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003; 460:167–179.

24. Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996; 206:33–36.

25. Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004; 74:2627–2642.

26. Ozawa T, Aoki Y, Ohtori S, Takahashi K, Chiba T, Ino H, et al. The dorsal portion of the lumbar intervertebral disc is innervated primarily by small peptide-containing dorsal root ganglion neurons in rats. Neurosci Lett. 2003; 344:65–67.

27. Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004; 355:45–48.

28. Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004; 108:237–247.

29. Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2008; 12:564–572.

30. Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004; 101:12706–12711.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download