Abstract
Purpose
Diabetic nephropathy is a serious complication of type 2 diabetes mellitus, and delaying the development of diabetic nephropathy in patients with diabetes mellitus is very important. In this study, we investigated inflammation, oxidative stress, and lipid metabolism to assess whether curcumin ameliorates diabetic nephropathy.
Materials and Methods
Animals were divided into three groups: Long-Evans-Tokushima-Otsuka rats for normal controls, Otsuka-Long-Evans-Tokushima Fatty (OLETF) rats for the diabetic group, and curcumin-treated (100 mg/kg/day) OLETF rats. We measured body and epididymal fat weights, and examined plasma glucose, adiponectin, and lipid profiles at 45 weeks. To confirm renal damage, we measured albumin-creatinine ratio, superoxide dismutase (SOD), and malondialdehyde (MDA) in urine samples. Glomerular basement membrane thickness and slit pore density were evaluated in the renal cortex tissue of rats. Furthermore, we conducted adenosine monophosphate-activated protein kinase (AMPK) signaling and oxidative stress-related nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling to investigate mechanisms of lipotoxicity in kidneys.
Diabetic nephropathy (DN) is the most common cause of progressive kidney disease, and is known to be a life-threatening complication of diabetes mellitus, showing high mortality around the world.12 In early stages of diabetic mellitus, blood glucose rises beyond the kidney's capacity to reabsorb glucose from the renal ultrafiltrate, such that glucose remains diluted in the fluid. As a result, glucose raises osmotic pressure and increases urine volume. Albuminuria in the progressive stage of diabetic mellitus has been considered to be a consequence of hyperglycemia and glomerular hyperfiltration, leading to basement membrane thickening, expansion of mesangium, and impaired glomerular sclerosis.34 Moreover, in uncontrolled type 2 diabetes mellitus, one easily finds elevated serum lipid profiles, such as total cholesterol (TC), triglyceride (TG), and free fatty acid (FFA).5 Excessive energy intake exceeds the storage capacity of adipose tissue, leading to the accumulation of excess energy as fat to ectopic sites.67 Consequently, lipid-mediated and increased oxidative stress by renal lipid accumulation may lead to kidney dysfunction.68
Considered a metabolic master switch, 5' adenosine monophosphate-activated protein kinase (AMPK) regulates several intracellular systems, including cellular uptake of glucose in the liver, skeletal muscle, and brain.910 It has also been newly identified as a regulator of renal hypertrophy in diabetes and has been shown to be inhibited in diabetic kidney.111 Several studies have revealed that AMPK signaling might play a role in renal cells.1213 Also, AMPK plays a key role in lipid metabolism, although the role of AMPK signaling in DN with ectopic lipid accumulation has not been well defined to date.
Reactive oxygen species (ROS) appear to play an important role in the progression of DN.2 Diabetic complications are caused by prolonged exposure to high glucose levels that lead to the mitochondrial overproduction of ROS.14 In DN, ROS leads to oxidative stress-related renal injuries, which are marked by the structural and functional changes in glomerular and renal tubular cells.1415 Normally, renal cells defend themselves against ROS damage with various antioxidants, such as superoxide dismutase (SOD), catalases, glutathione, and peroxiredoxins. Among these antioxidants, SOD is a powerful enzyme: transgenic overexpression SOD in diabetic mice reduced oxidative stress, albuminuria, and glomerular matrix.3 Also, several studies showed that increased lipid metabolism and ROS may be related to the activation of renal tissue damage.616 Specially, SOD and catalase are known to be regulated by the transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2).17 Nrf2 plays a key role in inhibiting oxidative stress and lipid accumulation in type 2 DN and high-fat diet.1819
Curcumin [1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadin-3,5-dione] is an active component in turmeric rhizomes (Curcuma Longa Linn), and is a major ingredient of spices, such as turmeric and curry. Yellowish curcumin has anti-oxidative, anti-carcinogenic, anti-inflammatory, anti-hyperlipidemic, and hypoglycemic properties.14 Curcumin has been extensively studied and found to be protective against neuropathy,20 nephropathy,2122 retinopathy,23 vascular disease,24 and pancreatic β-cell dysfunction25 in diabetic mellitus. In particular, the antioxidant effects of curcumin have been reported to be mediated by up-regulation of SOD;2627 moreover, curcumin is also closely associated with enhanced SOD activity and reducing intracellular ROS with aging.2829 Furthermore, curcumin activates Nrf2 to up-regulate enzymes involved in antioxidant defense, like SOD and heme oxygenase-1 (HO-1).3031 As for lipid metabolism, curcumin reduces lipidemia, cholesterol, and lipid peroxidation products in the blood and urine of diabetic mellitus.3233
With the properties described above, curcumin may reduce lipid metabolism and oxidative stress in DN. Therefore, we evaluated whether curcumin exerts therapeutic effects on lipid accumulation and induced oxidative stress related to AMPK activation and its downstream molecules in DN in rats.
All animal procedures were approved by the Institutional Animal Care and Use Committees of Yonsei University, Wonju, Korea (IRB No. 090422-2). At 25 weeks, 30 animals were divided into three groups: 10 male Long-Evans-Tokushima-Otsuka rats for the normal control group (CON) and 20 male Otsuka-Long-Evans-Tokushima Fatty (OLETF) (Otsuka Pharmaceutical, Tokushima, Japan) rats for diabetic control (DM) and curcumin-treated groups (CUR). In the diabetic CON group, one OLETF rat was excluded during the experiment period because of poor condition. In the end, a total of 29 rats were included in the experiment. The rats were housed at constant temperature (20–22℃) and humidity (50–60%) with a 12:12-h light/dark cycles with water and standard lab chow diet until 45 weeks of age. The experimental group received curcumin (100 mg/kg/day) and the diabetic group received saline through a 20-gauge feeding needle from 25 weeks to 45 weeks. The dosage of curcumin was adjusted for the large body surfaces and body weights of the obese OLETF rats, compared to those of other rat models,19 and for 20 weeks treatment. To create hyperglycemic conditions and induce diabetes in the type 2 diabetic models, a 30% sucrose solution was freely fed to the DM and CUR.
Body weight and blood glucose levels (Surestep; Lifescan Inc., Milpitas, CA, USA) were measured at 25 and 45 weeks, at which time an intra-peritoneal glucose tolerance test (IPGTT) and an intravenous insulin tolerance test (IVITT) were done. Indices of insulin resistance were calculated using the following formula: Kitt (rate constant for plasma glucose disappearance, %/min)=0.693/T1/2×100. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)=fasting insulin (µU/mL)×fasting glucose (mmol/mL)/22.5. Insulin secretion was calculated using HOMA-β=20×fasting insulin (µU/mL)/fasting glucose (mmol/L)-3.5.
At the end of 45 weeks, the rats were fasted overnight and then anesthetized with Zoletil® (Virbac Laboratories, Carros, France) by intra-peritoneal injection. Blood was collected via heart puncture with a needle into ethylenediaminetetraacetic acid-treated tube, and just before the time of death, a saline perfusion was performed, with an exception for the left kidney. The blood tubes were centrifuged, and clear plasma was stored in deep freezer (-80℃) until the measurement. Both kidneys and epididymal fat were extracted, and the left kidney was preserved using a quick freezing method with liquid nitrogen. One half of the right kidney was fixed with 4% paraformaldehyde for 48 hours and then embedded in paraffin for histological examination, while the remaining half was immediately treated with 3% glutaraldehyde at 4℃ for 24 hours to prepare for ultra-structural studies.
Blood lipid levels, including TC and TG, were determined with DRI-CHEM 3500i (FujiFilm, Tokyo, Japan).
Malondialdehyde (MDA) was measured by fluorometric high-performance liquid chromatography at the NeoDin Medical Institute (Seoul, Korea). Briefly, urine samples were treated with 0.1125 N PCA and 40 mM 2-thiobarbituric acid, and a derivative was formed by heating at 97℃ for 1 hour. The solution was then placed on ice for 20 minutes to stop the reaction. After 20 minutes, methanol and 20% trichloroacetic acid buffer were added. The samples were mixed and centrifuged at 13000 g for 6 minutes. The supernatant was then read using an insert vial and a fluorescence detector. The fluorescence detector was set at an excitation wave length of 525 nm and emission of 560 nm. The run time was 2 minutes, and the flow rate was 1 mL/min.
Twenty-four-hour urine samples were collected for the assessment of albumin, creatinine (Exocell Nepharat; Exocell Inc., Philadelphia, PA, USA), and urine SOD (Cayman Chemical Company, Ann Arbor, MI, USA). Plasma insulin (Shibayagi Co., Shibukawa, Japan), adiponectin (AdipoGen Inc., Seoul, Korea), and FFA (Abcam, Cambridge, MA, USA) were measured with enzyme-linked immunosorbent assay kits.
Paraffin embedded tissues were cut into 5-µm thick sections and stained with hematoxylin and eosin (H&E) stain. We examined these sections with an optical microscope that was equipped with a charge coupled device camera (Pulnix, Sunnyvale, CA, USA) in order to obtain pictures of the glomeruli, which were subsequently sent to a computer monitor. We measured 40 glomerular areas per rat using an image analysis system (GmbH, SIS, Minster, Germany). In addition, we calculated glomerular volume (Gv) by the Weibel and Gomez formula: 18 Gv=area 1.5×1.38/1.01 (1.38: shape coefficient, 1.01: size distribution coefficient).
Each specimen was prepared at all steps with an electron microscope reagent to make ultrathin sections, and examined under a transmission electron microscope (JEM-1200EX II, JEOL Ltd., Tokyo, Japan). Electron micrographs of five to ten glomeruli per kidney were randomly taken at 30000× for each rat. Photomicrographs of glomerular basement membrane (GBM) were also analyzed for the density of slit pores between the podocyte foot processes. The number of slit pores was enumerated and divided by the GBM length (mm) to derive linear density using an image analyzer (GmbH, SIS, Minster, Germany).
Renal cortex protein was extracted using PRO-PREPTM protein Extraction Solution (Intron Biotechnology, Seoul, Korea) by Tissue Lyser II (QIAGEN GmbH, Haan, Germany). All protein concentrations were measured with a Bio-Rad protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Thirty micrograms of each protein sample were run on an 8–12% sodium dodecyl sulfate-poly-acrylamide gel under denaturing conditions. The proteins were transferred onto a polyvinylidene difluoride membrane (Immobilon; Millipore, Bedford, MA, USA) for 90 minutes at 280 mA. The membranes were blocked by incubating the membranes with 5% skim milk or bovine serum albumin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 1 hour at room temperature in phosphate buffered saline with Tween 20 solution. The membrane was hybridized with anti-p-AMPK, anti-AMPK, and anti-p-ACC antibodies (Cell Signaling Technology Inc., Danvers, MA, USA), or anti-β-actin, anti-Nrf2, anti-Keap1, anti-HO-1, anti-vascular endothelial growth factor, anti-tumor growth factor-β1, anti-SREBP1, anti-SREBP2, and anti-adipose differentiation related protein (ADRP) antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in a blocking buffer overnight at 4℃. The filter was incubated with anti-rabbit or anti-mouse for 60 minutes at room temperature. Specific signals were detected using the electrochemiluminescence solution (Santa Cruz Biotechnology Inc.).
This assay was designed to measure lipid accumulation in kidney tissue. The reagent measured the concentration of glycerol released after lysing the cells and hydrolyzing the TG molecules. The TG concentration could then be determined from the glycerol values.
All results were presented as means and standard deviations. The data were analyzed by ANOVA or repeated measure ANOVA. Sheffé's post hoc test was performed to determine significant differences among the groups using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA). p<0.05 was considered statistically significant.
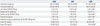
At the end of 45 weeks, the DM group exhibited significantly increased fasting blood sugar and significantly decreased insulin levels, compared to the CON group. Kitt, homeostatic model assessment beta cell function (HOMA-beta), and HOMA-IR values were calculated. Kitt and HOMA-beta values of the DM group were markedly lower than those of the CON group (p<0.05). No differences were observed in the HOMA-IR values among the three groups (Table 1). Changes in plasma glucose during the IVITT and the IPGTT in each group at 45 weeks of age are illustrated (Fig. 1). As expected, there were significant differences in IVITT and IPGTT between the CON and DM groups. However, the differences between DM and CUR groups in IVITT and IPGTT were not significant.
At 25 weeks of age, 24-hour urinary albumin excretion and albumin creatinine ratio (ACR) did not vary among the OLETF rat groups (data not shown). At 45 weeks of age, the 24-hour urinary ACR levels of the DM group increased, compared to the CON group, and those of the CUR group were significantly lower than those of the DM group (Fig. 2).
In representative images of glomerular H&E staining, the DM group showed glomerular hypertrophy, while the CUR group showed reductions in diabetic alterations in kidney at 45 weeks of age (Fig. 3A). Also, Gv significantly increased in the DM group, which curcumin treatment significantly decreased (Fig. 3C).
Next, we measured GBM thickness and podocyte morphometry for open slit pore number in each group. As expected, GBM thickness was increased in the DM group, and podocyte foot processes were effaced (Fig. 3B). The number of slit pores per unit length of GBM between the podocyte foot processes was significantly decreased in the DM group (p<0.05). In contrast to these changes, curcumin significantly ameliorated the diabetic condition (p<0.05) (Fig. 3D and E). In the curcumin treated group, GBM thickness was decreased, while there was less fading of the podocyte foot processes and the number of open slit pore was increased, compared to DM group.
Urine SOD and MDA levels from 24-hour urine samples were measured. In CUR group, SOD was significantly elevated compared to those in the untreated DM group. In addition, urine MDA, a known lipid-peroxidation related marker of oxidative stress, was significantly increased in the DM group, compared to the CON group. After curcumin treatment, oxidative stress was significantly decreased in the DM group (Fig. 4A and B). As shown in Fig. 4C and D, the expressions of Nrf2/Keap1 protein ratio and HO-1 protein were significantly increased in the CUR group, compared with the DM group. Nrf2 protein levels were increased, while Keap1 was decreased, in the DM group.
Prior to receiving curcumin treatment, the initial body weights of the DM group and CUR group at 25 weeks of age were significantly higher than those of the CON group. Twenty weeks later, there was no significant difference in final body weights among groups. In addition, during the experiment period, no difference was found in the amount of diet consumed between the DM group and CUR groups. After adjusting kidney and epididymal fat weights by body weight, it was found that the left and right kidney weights of the DM and CUR groups were markedly heavier than those of the CON group. However, the differences were not significant between those of the DM and CUR groups. Interestingly, the adjusted epididymal fat/body weight ratio (fat Wt/BW) of the CUR group was significantly lighter than those of the CON group. Along with decreased epididymal fat (Wt/BW) of the CUR group, the serum TC, TG, and FFA levels of the CUR group were significantly lower than those of the DM group at 45 weeks of age. Plasma adiponectin level of the CUR group was not different from that of the DM group. Meanwhile, adiponectin levels adjusted by epididymal fat Wt/BW of the CUR group were significantly higher than those of the DM group (Table 2, Fig. 5A).
We examined renal TG levels to measure lipid accumulation in the kidney after lysing the cells and hydrolyzing the TG molecules. Renal TG levels were significantly decreased in the curcumin treatment group (Fig. 5B). To identify lipid metabolism, we measured sterol regulatory element-binding protein-1 and 2, ADRP, 5' AMPK and its phosphorylation, and acetyl-CoA carboxylase (ACC) phosphorylation in the renal cortex. AMPK phosphorylation was reduced in the DM group and curcumin prevented the reduction of AMPK phosphorylation in the DM group. Phosphorylation of ACC resulted in decreased fatty acid transport and subsequent oxidation in renal cortex tissue by curcumin treatment (Fig. 5C and D). SREBP-1 and SREBP-2 were increased in the DM group. The CUR group had significantly reduced SREBP-1 and SREBP-2, compared to the DM group. Also, increased ADRP protein expression in the DM group was attenuated by curcumin treatment (Fig. 5E and F).
In the present study, we found that anti-oxidant enzymes were suppressed and lipid-related molecules were excessively expressed in the kidneys of type 2 diabetic OLETF rats. DN manifests primarily in clinical findings, such as albuminuria, wide GBM thickness, and a decreased number of open slit pores in podocytes. These renal injury conditions occurred usually at around 40 weeks after the onset of diabetes in OLETF rats.34 Furthermore, we demonstrated diabetes-induced oxidative damage to the kidneys as evidenced by changes in SOD and MDA with Nrf2 signaling related-molecules.35 Also, we identified ectopic lipid-related damage to the kidneys as evidenced by changes in lipid molecules with AMPK signaling related-proteins. All these alterations, which are manifestations in diabetic kidneys, were prevented with curcumin.
In this study, curcumin affected ROS, which plays a pivotal role in the pathophysiology of diabetic renal change. Hyperglycemia could induce ROS production and lipid peroxidation in a diabetic condition, leading to the development of DN.1436 The present study found that curcumin increased urinary SOD, a superoxide radical scavenger enzyme, while at the same time, decreased urinary MDA, a lipid peroxidation index. Thus, we speculate that curcumin might protect against DN via anti-oxidant mechanisms rather than glucose-related mechanisms.16 Especially, reduced expression of Nrf2 and increased oxidative stress have been observed in DN, and Keap1 is known to contribute to increased oxidative stress through negative regulation of Nrf2 activiry.183738 Reduced HO-1 expression in renal cortex tissue of diabetic mellitus might be related to decrease Nrf2 through induced ROS.30 In the present study, curcumin up-regulated HO-1 via activation of Nrf2, leading to attenuation of elevations of MDA, and ROS regulated with SOD in DN. Also, activation of Nrf2 may inhibit lipid accumulation in the kidneys of type 2 diabetic OLETF rats.1931 These anti-oxidative effects of curcumin could attenuate increased albuminuria, GBM thickness, or damage to the podocyte foot process by relieving hyperlipidemia.3940
An alternate mechanism by which curcumin may exert effects on diabetic complications is through lipid metabolism. Previous studies have suggested that improvement of hyperlipidemia is achieved with curcumin,3233 and decreased cholesterol and TG levels could be related to the inhibition of DN. Like type 2 diabetes mellitus, high levels of cholesterol, TG, and free fatty acid could stimulate ectopic lipid accumulation in the organs, except adipose tissue, and especially lipid accumulation of renal tissue could be a crucial factor in the development of chronic kidney disease.633 Indeed, several studies have shown the presence of lipid deposits in the kidneys of diabetic models.541 AMPK phosphorylation was reduced in the diabetic group,533 and curcumin treatment activated AMPK phosphorylation, showing that it may control the AMPK activity. Similar to a previous study, SREBP-1, which activates fatty acid synthesis, and SREBP-2, which activates cholesterol biosynthesis, were increased in the DN; however, curcumin significantly reduced SREBP-1 and SREBP-2 in the present study. Also, ADRP, a marker of lipid droplets, was increased in the diabetic group, although it was attenuated by curcumin treatment.3342 One of the key pathways in AMPK's regulation of fatty acid oxidation is the phosphorylation and inactivation of ACC. ACC converts acetyl-CoA to malonyl-CoA, and then blocks fatty acid oxidation. Phosphorylation of ACC, therefore, results in decreased fatty acid transport and subsequent oxidation by curcumin. Our study outlined a possible pathway of the effects of curcumin on DN via AMPK-mediated regulation of lipid synthesis. This is consistent with previous results showing curcumin inhibits lipid accumulation in the kidneys of diabetic rats.33
In conclusion, curcumin decreased TC, TG, and FFA in serum and decreased lipid accumulation in the kidneys through AMPK signaling. Also, it decreased albuminuria by ameliorating pathophysiologic changes and oxidative stress on the glomerulus through Nrf2 signaling. Thus, we suggest that curcumin may holds protective or ameliorating effects against type 2 DN through anti-oxidative and hypo-lipidemic effects.
Figures and Tables
Fig. 1
Effect of curcumin on insulin tolerance and glucose tolerance tests. (A) At 45 weeks of age, the plasma glucose level of the DM group was significantly increased, compared to the CON group, using the intravenous insulin tolerance test, while glucose levels of the CUR group were not significant, compared to the DM group. (B) Using the intraperitoneal glucose tolerance test, the plasma glucose level of the DM group was found to be significantly decreased, compared to the CON group; however, such changes in glucose levels were not shown in the CUR group, compared to the DM group. Data expressed as mean±SD. *p<0.05 vs. CON. CON (•), control group; DM (▪), diabetic group; CUR (▴), curcumin-treated (100 mg/kg, p.o.) diabetic group.

Fig. 2
Effect of curcumin on urine albumin levels and 24-hour albumin/creatinine ratio (ACR). (A) At 45 weeks of age, urinary albumin levels of the CUR group in 24-hour urine samples were significantly decreased, compared to the DM group. (B) 24-hour ACR was also significantly decreased, compared to the DM group. Data expressed as mean±SD. *p<0.05 vs. CON, †p<0.05 vs. DM. CON, control group; DM, diabetic group; CUR, curcumin-treated (100 mg/kg, p.o.) diabetic group.

Fig. 3
Effect of curcumin on kidney histological morphology changes. (A) Representative image of glomerular hematoxylin and eosin (H&E)-stained sections. (B) Representative electron photomicrographs show glomerular basement membrane (GBM) thickness and open slit pores (arrows). (C) Glomerular volume. Differences in glomerular volume were not significant among these three groups. (D) GBM thickness. DM group shows significant glomerular thickening, compared with the CON group, while the CUR group shows significant reductions in diabetic alterations in kidneys at 45 weeks of age. (E) The number of open slit pores between podocyte foot processes. Glomerular injury decreased after treatment with curcumin, compared to the DM group. Data express mean±SD. *p<0.05 vs. CON, †p<0.05 vs. DM. H&E staining scale bar, 100 µm, ×400. EM scale bar, 100 µm, ×30000. CON, control group; DM, diabetic group; CUR, curcumin-treated (100 mg/kg, p.o.) diabetic group.
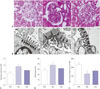
Fig. 4
Effect of curcumin on urine SOD and MDA, Nrf2/Keap1, and HO-1 protein expression in the urine or renal cortex. (A) Urinary SOD was significantly increased in the CUR group, compared to the DM group, at 45 weeks of age. (B) Urinary MDA significantly decreased in the CUR group, compared with the DM group. (C) Representative pictures of Nrf2/Keap1 protein ratio and HO-1 expression in renal kidney cortex of 45 weeks of age. (D) Reduced Nrf2/Keap1 protein ratio and HO-1 protein expression related to renal oxidative stress, which were significantly increased in the CUR group. Data expressed as mean±SD. *p<0.05 vs. CON, †p<0.05 vs. DM. CON, control group; DM, diabetic group; CUR, curcumin-treated (100 mg/kg, p.o.) diabetic group; SOD, superoxide dismutase; MDA, malondialdehyde; Nrf2, nuclear factor (erythroid-derived 2)-like 2; HO-1, heme oxygenase-1.
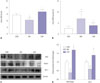
Fig. 5
Effect of curcumin on FFA, renal triglyceride (TG), AMPK, ACC, SREBP-1, SREBP-2, and ADRP protein expressions in renal cortex. (A) Serum FFA level. It was significantly increased in the DM group at 45 weeks of age, but it was significantly decreased by curcumin treatment. (B) Renal TG level. It tended to increase in the DM group (p=0.055), and curcumin significantly reduced renal TG. (C) Representative Western blots depicting protein abundance of the phosphorylated AMPK and ACC in the renal cortex. (D) The DM group showed significantly decreased phosphorylated AMPK and ACC expression in the renal cortex. These changes were reversed upon treatment with curcumin. (E) Representative Western blots depicting protein abundance of SREBP-1, SREBP-2, and ADRP in the renal tissues. (F) The DM group showed significantly increased SREBP-1, SREBP-2, and ADRP expressions in renal cortex. These changes were reversed upon treatment with curcumin. Data expressed as mean±SD. *p<0.05 vs. CON, †p<0.05 vs. DM. CON, control group; DM, diabetic group; CUR, curcumin-treated (100 mg/kg, p.o.) diabetic group; FFA, free fatty acid; AMPK, adenosine monophosphate-activated protein kinase; ACC, acetyl-CoA carboxylase; ADRP, adipose differentiation related protein.
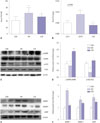
Table 1
Blood Glucose and Insulin Test

CON, control; DM, diabetes; CUR, curcumin (100 mg/kg, p.o.) treated DM; FBS, fasting blood sugar; Kitt, rate constant for plasma glucose disappearance; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostatic model assessment beta cell function.
The values are the mean±SD.
*p<0.05 vs. CON.
Table 2
Body and Organ Weights and Lipid Profiles
ACKNOWLEDGEMENTS
This work was supported in part by the Yonsei University Research Fund of 2009 and also was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2044121).
References
1. Kume S, Koya D, Uzu T, Maegawa H. Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy. Biomed Res Int. 2014; 2014:315494.

2. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28:164–176.

3. Zelmanovitz T, Gerchman F, Balthazar AP, Thomazelli FC, Matos JD, Canani LH. Diabetic nephropathy. Diabetol Metab Syndr. 2009; 1:10.

4. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008; 233:4–11.

5. Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005; 54:2328–2335.

6. Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, et al. Ectopic lipid accumulation: a potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013; 95:1971–1979.

7. de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014; 2:417–426.

8. Tesch GH, Lim AK. Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2011; 300:F301–F310.
9. Hasenour CM, Berglund ED, Wasserman DH. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol Cell Endocrinol. 2013; 366:152–162.

10. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012; 13:251–262.

11. Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007; 292:F617–F627.

12. Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, et al. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal. 2010; 22:65–70.

13. Lee HJ, Mariappan MM, Feliers D, Cavaglieri RC, Sataranatarajan K, Abboud HE, et al. Hydrogen sulfide inhibits high glucose-induced matrix protein synthesis by activating AMP-activated protein kinase in renal epithelial cells. J Biol Chem. 2012; 287:4451–4461.

14. Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013; 2013:636053.

15. Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, et al. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. 2013; 18:756–763.

16. DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004; 53:762–768.

17. Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011; 31:58–66.

18. Nakai K, Fujii H, Kono K, Goto S, Kitazawa R, Kitazawa S, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014; 27:586–595.

19. Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008; 325:655–664.

20. Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006; 536:256–261.

21. Huang J, Huang K, Lan T, Xie X, Shen X, Liu P, et al. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol. 2013; 365:231–240.

22. Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006; 33:940–945.

23. Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011; 27:123–130.

24. Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med. 2010; 10:57.

25. Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007; 577:183–191.

26. Sreejayan , Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994; 46:1013–1016.

27. Buyuklu M, Kandemir FM, Ozkaraca M, Set T, Bakirci EM, Topal E. Protective effect of curcumin against contrast induced nephropathy in rat kidney: what is happening to oxidative stress, inflammation, autophagy and apoptosis? Eur Rev Med Pharmacol Sci. 2014; 18:461–470.
28. Suckow BK, Suckow MA. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. Int J Biomed Sci. 2006; 2:402–405.
29. Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, et al. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr). 2013; 35:1133–1142.

30. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003; 371(Pt 3):887–895.

31. Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013; 39:101–121.

32. Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007; 29:881–889.

33. Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Harima M, Suzuki K, et al. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J Nutr Biochem. 2013; 24:796–802.

34. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992; 41:1422–1428.

35. Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition. 2009; 25:964–972.

36. Asai A, Nakagawa K, Miyazawa T. Antioxidative effects of turmeric, rosemary and capsicum extracts on membrane phospholipid peroxidation and liver lipid metabolism in mice. Biosci Biotechnol Biochem. 1999; 63:2118–2122.

37. Dadras F, Khoshjou F. NF-E2-related factor 2 and its role in diabetic nephropathy. Iran J Kidney Dis. 2013; 7:346–351.
38. Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010; 59:850–860.

39. Smyth R, Lane CS, Ashiq R, Turton JA, Clarke CJ, Dare TO, et al. Proteomic investigation of urinary markers of carbon-tetrachloride-induced hepatic fibrosis in the Hanover Wistar rat. Cell Biol Toxicol. 2009; 25:499–512.

40. Liu D, Huang P, Li X, Ge M, Luo G, Hei Z. Using inflammatory and oxidative biomarkers in urine to predict early acute kidney injury in patients undergoing liver transplantation. Biomarkers. 2014; 19:424–429.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download