Abstract
Purpose
Left ventricular (LV) filling pressure affects atrial fibrillation (AF) recurrence. We investigated the relationship between diastolic dysfunction and AF recurrence after cardioversion, and whether LV filling pressure was predictive of AF recurrence.
Materials and Methods
Sixty-six patients (mean 58±12 years) with newly diagnosed persistent AF were retrospectively enrolled. We excluded patients with left atrial (LA) diameters larger than 50 mm, thereby isolating the effect of LV filling pressure. We evaluated the differences between the patients with (group 1) and without AF recurrence (group 2).
Results
Group 1 showed increased LA volume index (LAVI) and E/e' compared to group 2 (p<0.05). During a mean follow-up period of 25±19 months, AF recurrence after cardioversion was 60.6% (40/66). The area under the receiver operating characteristics curve of E/e' for AF recurrence was 0.780 [95% confidence interval (CI): 0.657-0.903], and the optimal cut-off value of the E/e' was 9.15 with 75.0% of sensitivity and 73.1% of specificity. A Kaplan-Meier survival curve showed that the cumulative recurrence-free survival rate was significantly lower in patients with higher LV filling pressure (E/e'>9.15) compared with patients with lower LV filling pressure (E/e'≤9.15) (log rank p=0.008). Cox regression analysis revealed that E/e' [hazards ratio (HR): 1.100, 95% CI: 1.017-1.190] and LAVI (HR: 1.042, 95% CI: 1.002-1.084) were independent predictors for AF recurrence after cardioversion.
Atrial fibrillation (AF) is the most common arrhythmia and is related with cardiovascular disorders, including heart failure and stroke, and doubles the associated mortality rate.1 Several studies have indicated diastolic dysfunction to be an independent predictor of AF.2 The presence and severity of diastolic dysfunction might be associated with the left atrium (LA) substrate of AF and progressive atrial mechanical remodeling due to increased LA pressure.3 Increased left ventricular (LV) filling pressure has been linked with all-cause mortality, an increased frequency of LA appendage thrombus, and stroke in non-valvular AF patients.4
We hypothesized that the AF recurrence is associated with the degree of diastolic dysfunction in patients with persistent AF after cardioversion. Several studies have demonstrated that increased LV filling pressure may affect AF recurrence after cardioversion.24 Therefore, this study set out to determine whether increased LV filling pressure is associated with an increased risk of AF recurrence after cardioversion in patients with persistent AF. In contrast to previous studies, to limit the effect of LA structural remodeling which is in the pathogenesis of AF, we enrolled patients without extreme LA enlargement. A limited number of studies have investigated how diastolic parameters may predict AF recurrence after cardioversion by using echocardiography. Here, we investigated the predictors of AF recurrence in patients undergoing continued antiarrhythmic drug therapy after cardioversion.
The study retrospectively enrolled 66 patients (57 males, mean 58±12 years) with newly diagnosed non-valvular, lone, and persistent AF from January 2009 to December 2012 at the Gangnam Severance Hospital, Yonsei University College of Medicine. Persistent AF was defined as continuous AF sustained greater than 7 days, according to the expert consensus statement.5 For patients with newly diagnosed AF, heart rate was controlled below 110 beats/minute and maintained by using beta blockers or calcium channel blockers. After heart rate control, pre-treatment with flecainide for 2 weeks was performed in all patients before electrical cardioversion. Those patients who were converted to sinus rhythm by pretreatment with flecainide were labeled as the chemical cardioversion group. Electrical cardioversion was performed in the remaining patients who were not converted to sinus rhythm by flecainide treatment. These patients were labeled as the electrical cardioversion group. All 66 patients were converted to sinus rhythm by either flecainide treatment or electrical cardioversion. After cardioversion, flecainide was administered continuously in order to maintain sinus rhythm. All patients who underwent electrical cardioversion had taken oral anticoagulation with vitamin K antagonists such as warfarin for 4 weeks and had maintained optimal prothrombin time (PT) international normalized ratio (INR) range of 2.0 to 3.0 before the cardioversion. Exclusion criteria were: 1) LV ejection fraction (EF) <50%, 2) LA anterior-posterior (AP) dimension >50 mm, and 3) known coronary artery disease (CAD) or suspected CAD. The patients were divided into two groups according to AF recurrence: group 1, with AF recurrence (n=40); and group 2, without AF recurrence (n=26). Electronic medical records were reviewed, and pertinent data points were recorded. All patients provided written, informed consent.
After rate control, two-dimensional transthoracic echocardiography (TTE) was performed. All the echocardiographic studies were performed using an iE33 (Philips Ultrasound, Bothell, WA, USA) with an S3 probe. Comprehensive echo-Doppler and M-mode evaluation were assessed in all patients before cardioversion. Left ventricle wall thicknesses was measured during end-diastole phases. LA AP dimension was measured at end-systole from the parasternal long axis view. All measurements were done according to current American Society of Echocardiography guidelines.6 The modified Simpson's rule was used to calculate LV volumes and EF from apical 2- and 4-chamber views. The prolate ellipse method was used to calculate LA volume from apical 4-chamber and parasternal long-axis views at ventricular end-systole, then LA volumes were indexed to body surface area.
Peak early (E) and late (A) diastolic mitral inflow velocities were measured in apical 4-chamber view. Tissue Doppler interrogation was done in septal mitral annulus in apical 4-chamber view, and then peak systolic mitral annulus velocity and early diastolic mitral annulus peak velocity (e') were measured, and the ratio of E/e' was calculated. Pulsed Doppler and pulsed tissue Doppler parameters were measured as the average of three cardiac cycles, and the R-R intervals were relatively regular except during lasting atrial fibrillation.
After the diagnosis of AF, patients were treated by flecainide for two weeks. If sinus rhythm was not achieved after chemical cardioversion, electrical cardioversion was performed. All patients were scheduled for regular follow-up visits after cardioversion, including clinical examination, and 12-lead electrocardiography (ECG) every 3 months and Holter monitoring every 6 months. In the case of symptom recurrence between follow-up visits, patients were evaluated by additional clinical examination, ECG, and Holter monitoring. AF recurrence after cardioversion was defined as any documented supraventricular tachyarrhythmia, such as AF, atrial flutter, or atrial tachycardia episode, lasting >30 seconds.
Continuous variables that are normally distributed are reported as mean±SD or 95% confidence interval (CI). Student t-test was used to compare the means of continuous variables that were approximately normally distributed between the two groups. Continuous variables that were not normally distributed are reported as median (25-75 percentile range) and are compared using the Kruskal-Wallis test. Normality was determined using the Kolmogorov-Smirnov goodness-of-fit test. Categorical variables are reported as count (percentage) and are compared using Fisher's exact test.
Independent predictors of AF recurrence after cardioversion were assessed using univariate Cox proportional hazards regression models, and multivariable models were assessed based on the results of univariate models. Multivariate analysis (stepwise forward and enter method) was done with variable with p<0.05 in univariate analysis. Differences in cumulative event-free survival rate between patients with and without elevated LV filling pressure were explored using the Kaplan-Meier method followed by log-rank test. Receiver operating characteristic (ROC) analysis was performed to evaluate the values for predicting AF recurrence and identify the optimal cut-off values for E/e' level. A p-value of ≤0.05 was considered statistically significant.
The SPSS statistical package (IBM, Markham, Canada) was used to perform all statistical evaluations.
The baseline characteristics of the patients are presented in Table 1. There were no differences in the age, gender, hypertension, diabetes, dyslipidemia, stroke, smoking and CHA2DS2-Vasc score between the groups. There were no differences in the LA AP dimension, LV mass index, or LV EF between the groups. Group 1 had an increased LA volume index (LAVI; 30.30±8.32 mL/m2 vs. 25.47±7.75 mL/m2, p=0.021) and E/e' (10.9±3.7 vs. 8.3±2.3, p=0.002) compared with group 2. The difference of E/e' between two groups is described with representative cases in Fig. 1, and depicted by scatter plots in Fig. 2. There were no significant difference in the prescribed medications such as beta blocker, calcium channel blocker, angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker.
The conversion to sinus rhythm during pre-treatment with flecainide was achieved in 39/66 patients (59.1%). Electrical cardioversion was carried out in the remaining patients, 27/66 (40.9%). All patients were converted to sinus rhythm with either chemical or electrical cardioversion. During a mean follow-up period of 25±19 months, the recurrence rate of AF after cardioversion was 60.61% (40/66; 48.7% vs. 77.8% in chemical cardioversion vs. electrical cardioversion). The chemical cardioversion group had a decreased LA volume index (47.66 mL/m2 vs. 65.44 mL/m2, p<0.001) compared to the electrical cardioversion group. There was no difference in the value of E/e' (9.7±3.3 vs. 10.6±4.1, p=0.351) between these two groups.
The area under the ROC curve of E/e' for AF recurrence was 0.780 (95% CI: 0.657-0.903). ROC analysis showed the optimal cut-off value for the E/e' for predicting AF recurrence level to be 9.15 with 75.0% of sensitivity and 73.1% of specificity (Fig. 3).
Univariate Cox proportional hazards regression analysis showed that LAVI [hazards ratio (HR): 1.045, 95% CI: 1.007-1.085, p=0.020], LV mass index (HR: 1.022, 95% CI: 1.001-1.042, p=0.036) and the ratio of E/e' (HR: 1.113, 95% CI: 1.031-1.202, p=0.006) were significant predictors of AF recurrence after cardioversion. Multivariate Cox proportional hazards regression analysis with stepwise forward method revealed that E/e' (HR: 1.100, 95% CI: 1.017-1.190, p=0.017) and LAVI (HR: 1.042, 95% CI: 1.002-1.084, p=0.042) were independent predictors for AF recurrence after cardioversion (Table 2 and 3). Fig. 4 shows the Kaplan-Meier survival curves for AF recurrence with or without elevated LV filling pressure. The cumulative recurrence-free survival rate was significantly lower in patients with elevated LV filling pressure (E/e'>9.15) than patients without elevated LV filling pressure (E/e'≤9.15) (log rank p=0.008).
This study investigated the relationship between LV filling pressure and AF recurrence after cardioversion. In line with previous reports, overall AF recurrence rate in our study population was 60.6% (40/66).78 Diastolic dysfunction is linked with poorer cardiovascular outcomes, development of AF, more severe AF symptoms, as well known.9 The main finding of this study was that elevated LV filling pressure, as indicated by the increased E/e' ratio, was correlated significantly with AF recurrence after cardioversion. Our present study enrolled only patients who were newly diagnosed with AF in the absence of extremely enlarged LA. In this way, we were able to more effectively isolate the effect of LV filling pressure on AF recurrence. The results suggested that elevated LV filling pressure may predict the outcome after cardioversion in patients with persistent AF
Evaluation of diastolic function involves integration of multiple echocardiographic parameters. Diastolic dysfunction is characterized by a progressive decrease in LV compliance with corresponding impairment in myocardial relaxation, resulting in elevated LV end diastolic pressure despite normal end diastolic volume.2 Clinically useful parameters, which reflect the pressure gradient between the LA and LV, include mitral inflow Doppler patterns of early filling peak velocity (E), atrial peak velocity (A), the E/A ratio, and deceleration time (DT). These transmitral flow parameters are affected by loading status, and DT varies as it is dependent on the cardiac cycle length. Therefore, they need to be considered in combination with tissue Doppler imaging, which is less dependent on preload. Since, atrial systolic waves (A wave, atrial peak velocity) are lost in AF patients, it is difficult to evaluate diastolic function based on transmitral flow velocity.
Mitral annular velocity (e'), determined by pulsed wave tissue Doppler, is a relatively preload-independent variable, and has been demonstrated to be an excellent marker of LV relaxation.10 Therefore, E/e' reflects the LV filling pressure and is useful for evaluating diastolic function. Indeed, several studies have reported that E/e' is associated with LV filling pressure, even in AF patients, with sensitivities >70% and specificities >90%.11 Furthermore, Okura, et al.9 reported that E/e' was a strong predictor for heart failure in AF patients. In our study, the ratio of E/e' was a significant independent predictor of AF recurrence after cardioversion, indicating that LV filling pressure may be associated with AF recurrence. Since estimating E/e' during AF is less accurate than the same measure taken during sinus rhythm, we measured these parameters while the R-R intervals were relatively regular after rate control.
The association between the recurrence of AF after cardioversion and echocardiographic parameters reflecting diastolic dysfunction remains inadequately assessed. In the present study, we found that LV filling pressure was significantly correlated with AF recurrence after cardioversion.
When LV compliance is reduced, pressure essentially backs up, causing an increase in LA pressure. This atrial pressure overload leads to atrial electrical and structural remodeling including atrial stretching, dilatation and fibrosis. These changes provide a vulnerable substrate for AF.12 The mechanism of AF initiation and recurrence in patients with diastolic dysfunction stems from this progressive remodeling.313 Longterm volume overload could be predicted by both the LAVI and E/e', while long-term pressure overload of the LA leads to progressive LA enlargement and electrical instability, which reflect the severity of diastolic dysfunction. Therefore, AF recurrence after cardioversion is associated with elevated LV filling pressure and LA remodeling. Some studies have demonstrated LAVI to be an independent predictor of AF recurrence.14 The duration of AF reflects the degree of atrial remodeling and has also been demonstrated to be an important predictor of the success of cardioversion.14
Some studies have demonstrated that diastolic dysfunction might predict AF recurrence after cardioversion therapies.24 The LA structural remodeling reflects the chronicity of exposure to abnormal filling pressures as consequence of diastolic dysfunction.14 Caputo, et al.4 identified an enlarged LA volume and an elevated E/e' as predictors of AF recurrence after electrical cardioversion. However, they enrolled AF patients with no regard to LA size. This resulted in their study population to include patients with extremely enlarged atria. It is highly likely that elevated LV filling pressure, resulted from diastolic dysfunction, might cause the intrinsic pathologic physiologic change of LA before LA enlargement. LA dysfunction had been observed before LA enlargement in patients with paroxysmal AF, and this might be due to the reduction of booster pump function of LA, such as intrinsic active relaxation and contractility rather than conduit function.1516 Our study enrolled only patients who were newly diagnosed with AF in the absence of extremely enlarged LA. In these patients, the atria have not been given ample time to structurally remodel, thus resulting in an unchanged LA size.
Importantly, several studies have reported an LA diameter above 50 mm predicts the AF recurrence.17 By design, our study excluded patients with LA diameters larger than 50 mm in order to avoid the associated effects on AF recurrence. In this way, we could more effectively isolate the effect of LV filling pressure on AF recurrence, and found that E/e' was an independent predictor for AF recurrence after cardioversion, and an elevated E/e' was associated with poorer outcomes after cardioversion in patients with persistent AF patients. Therefore, we conclude that LV filling pressure, estimated by E/e', is predictive of AF recurrence after cardioversion even in the absence of extreme atrial enlargement.
It is important to manage diastolic dysfunction with optimal medical therapy. We can possibly do more intensive observation of symptoms and volume status of the patients with higher E/e' and without extreme LA enlargemt, and more aggressive medical treatment. The elevated LV filling pressure could be an early sign of left atrial pathological process before LA remodeling, presented as LA enlargement. Based on the result of this study, we carefully suggest that early medical intervention could improve the clinical prognosis regarding AF recurrences before the extreme LA enlargement observed by TTE. Proper management can attenuate the structural and electrical remodeling of the atria, typically associated with diastolic dysfunction, thereby limiting AF recurrence after cardioversion. Therefore, medical therapy targeted at reducing LV filling pressure is important before and after cardioversion. Renin-angiotensin system (RAS) inhibitors, such as angiotensinconverting enzyme inhibitor or angiotensin ll receptor blocker might reduce LV filling pressure. These medications may attenuate structural remodeling and electrical remodeling, and subsequently the recurrence of AF, by promoting the regression of atrial fibrosis.18 Indeed, Fukuda, et al.19 demonstrated that E/e' was higher in non-RAS inhibitor medication group than RAS inhibitor medication group. Although two prospective studies reported that RAS blockers could not suppress AF recurrence,2021 Ishikawa, et al.22 demonstrated that RAS inhibitor significantly reduced the AF recurrence after pulmonary vein isolation. Generally, therefore, these previous studies support that these drugs might reduce AF recurrence by decreasing LV filing pressure, the primary risk factor for AF recurrence assessed in this study. Based on the present results, further assessment of LV filling pressure as a valuable predictor of clinical outcomes associated with AF recurrence may help evaluate the effectiveness of these drugs. Moreover, a prospective study is needed to evaluate whether medical therapies aimed at lowering LV filling pressure could reduce AF recurrence after cardioversion and improve clinical outcomes in AF patients.
The present study has several limitations. This study was performed at a tertiary referral hospital, and there might be some selection and referral bias. The relatively small number of enrolled patients in this study is also a limitation. Additional echocardiographic parameters, such as left atrial appendage velocities using transesophageal echocardiography, were not considered in this study. Until now, there is no established method for evaluating LV diastolic function in AF patients, although several studies have reported clinical usefulness of E/e' to estimate LV filling pressure in this population.9 Therefore, we could not identify an association between diastolic dysfunction and AF recurrence. Additionally, Doppler parameters measured during AF could be less accurate than in the sinus rhythm, although we minimized this complication by measuring these parameters while the R-R intervals were relatively regular. Our study indicated that the echocardiographic parameters, indicating diastolic function such as E/e' ratio, can be reasonably assessed in AF patients. We think that more prospective studies are needed to firmly confirm the relationship between the medical treatment of diastolic dysfunction and the improvement of clinical prognosis regarding AF recurrences.
Our study provides data that support the assessment of LV filling pressure to predict clinical outcomes after cardioversion. The presence of LV diastolic dysfunction is a crucial mediator of AF development and recurrence. After cardioversion, patients with an elevated LV filling pressure, estimated by the E/e' ratio, had an increased AF recurrence rate without extreme LA enlargement. These results suggest that elevated LV filling pressure might predict the outcome after cardioversion of AF regardless of the degree of mechanical atrial remodeling. Therefore, it is important to manage diastolic dysfunction, including reduction of LV filling pressure, in order to reduce the AF recurrence rate and improve the outcome in patients with persistent AF after cardioversion. For early detected AF patients without extreme LA enlargement, reduction of LV filling pressure after cardioversion might decrease the risk of AF recurrence.
Figures and Tables
Fig. 1
The representative cases which reveal the difference of E/e' between two groups. (A) In patients without recurrence, early (E) diastolic mitral inflow peak velocity and early diastolic mitral annulus peak velocity (e') were measured, and the ratio of E/e' was calculated. (B) In patients with recurrence, the ratio of E/e' was calculated by the same way.
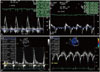
Fig. 2
The scatter plots of E/e' with or without AF recurrence. AF, atrial fibrillation; E/e', the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e').

Fig. 3
Receiver operating characteristic (ROC) curves for E/e' for AF recurrence after cardioversion. Area under the ROC curve for E/e' was 0.780 (95% confidence interval: 0.657-0.903, p<0.001). AF, atrial fibrillation; E/e', the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e').

Fig. 4
The Kaplan-Meier survival curves for AF recurrence in patients after cardioversion with or without increased LV filling pressure. AF, atrial fibrillation; E/e', the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e'); LV, left ventricle.

Table 1
Baseline Characteristics

LA, left atrium; LV, left ventricle; EF, ejection fraction; E/e', the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e'); DT, deceleration time; BB, beta blocker; CCB, calcium channel blocker; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin ll receptor blocker.
Table 2
Univariate Cox Proportional Hazards Regression Analysis for Predicting AF Recurrence after Cardioversion
Table 3
Multivariate Cox Proportional Hazards Regression Analysis for Predicting AF Recurrence after Cardioversion

ACKNOWLEDGEMENTS
We thank the study participants and supporting medical staffs for making this study possible.
References
1. European Heart Rhythm Association. European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31:2369–2429.
2. Melduni RM, Cullen MW. Role of left ventricular diastolic dysfunction in predicting atrial fibrillation recurrence after successful electrical cardioversion. J Atr Fibrillation. 2012; 5:87–94.
3. Huang JL, Tai CT, Lin YJ, Ting CT, Chen YT, Chang MS, et al. The mechanisms of an increased dominant frequency in the left atrial posterior wall during atrial fibrillation in acute atrial dilatation. J Cardiovasc Electrophysiol. 2006; 17:178–188.

4. Caputo M, Urselli R, Capati E, Navarri R, Sinesi L, Furiozzi F, et al. Usefulness of left ventricular diastolic dysfunction assessed by pulsed tissue Doppler imaging as a predictor of atrial fibrillation recurrence after successful electrical cardioversion. Am J Cardiol. 2011; 108:698–704.

5. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269–e367.
6. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.

7. Prystowsky EN, Benson DW Jr, Fuster V, Hart RG, Kay GN, Myerburg RJ, et al. Management of patients with atrial fibrillation. A statement for healthcare professionals. From the subcommittee on electrocardiography and electrophysiology, American Heart Association. Circulation. 1996; 93:1262–1277.
8. Kim H, Lee JP, Yoon HJ, Park HS, Cho YK, Nam CW, et al. Association between Doppler flow of atrial fibrillatory contraction and recurrence of atrial fibrillation after electrical cardioversion. J Am Soc Echocardiogr. 2014; 27:1107–1112.

9. Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, et al. Tissue Doppler-derived index of left ventricular filling pressure, E/E', predicts survival of patients with non-valvular atrial fibrillation. Heart. 2006; 92:1248–1252.

10. Bolognesi R, Tsialtas D, Barilli AL, Manca C, Zeppellini R, Javernaro A, et al. Detection of early abnormalities of left ventricular function by hemodynamic, echo-tissue Doppler imaging, and mitral Doppler flow techniques in patients with coronary artery disease and normal ejection fraction. J Am Soc Echocardiogr. 2001; 14:764–772.

11. Watanabe T, Iwai-Takano M, Oikawa M, Yamaki T, Yaoita H, Maruyama Y. Optimal noninvasive assessment of diastolic heart failure in patients with atrial fibrillation: comparison of tissue doppler echocardiography, left atrium size, and brain natriuretic peptide. J Am Soc Echocardiogr. 2008; 21:689–696.

12. Khan A, Moe GW, Nili N, Rezaei E, Eskandarian M, Butany J, et al. The cardiac atria are chambers of active remodeling and dynamic collagen turnover during evolving heart failure. J Am Coll Cardiol. 2004; 43:68–76.

13. Chin JY, Youn HJ. The effect of ablation for paroxysmal atrial fibrillation on left atrial volume and function: a one-year follow-up study. Yonsei Med J. 2014; 55:895–903.

14. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002; 90:1284–1289.

15. Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. 2012; 13:227–234.

16. Lancellotti P, Henri C. The left atrium: an old 'barometer' which can reveal great secrets. Eur J Heart Fail. 2014; 16:1047–1048.

17. Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. J Am Coll Cardiol. 2005; 45:2026–2033.

18. Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010; 55:2299–2307.

19. Fukuda Y, Fukuda N, Morishita S, Tamura Y. Preventive effect of renin-angiotensin system inhibitor on left atrial remodelling in patients with chronic atrial fibrillation: long-term echocardiographic study. Eur J Echocardiogr. 2011; 12:278–282.
20. ACTIVE I Investigators. Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011; 364:928–938.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download