Abstract
Purpose
To compare the effectiveness of device closure and medical therapy in prevention of recurrent embolic event in the Korean population with cryptogenic stroke and patent foramen ovale (PFO).
Materials and Methods
Consecutive 164 patients (men: 126 patients, mean age: 48.1 years, closure group: 72 patients, medical group: 92 patients) were enrolled. The primary end point was a composite of death, stroke, transient ischemic attack (TIA), or peripheral embolism.
Results
Baseline characteristics were similar in the two groups, except age, which was higher in the medical group (45.3±9.8 vs. 50.2±6.1, p<0.0001), and risk of paradoxical embolism score, which was higher in the closure group (6.2±1.6 vs. 5.7±1.3, p=0.026). On echocardiography, large right-to-left shunt (81.9% vs. 63.0%, p=0.009) and shunt at rest/septal hypermobility (61.1% vs. 23.9%, p<0.0001) were more common in the closure group. The device was successfully implanted in 71 (98.6%) patients. The primary end point occurred in 2 patients (2 TIA, 2.8%) in the closure group and in 2 (1 death, 1 stroke, 2.2%) in the medical group. Event-free survival rate did not differ between the two groups.
Conclusion
Compared to medical therapy, device closure of PFO in patients with cryptogenic stroke did not show difference in reduction of recurrent embolic events in the real world's setting. However, considering high risk of echocardiographic findings in the closure group, further investigation of the role of PFO closure in the Asian population is needed.
Patent foramen ovale (PFO) has been recognized as a possible cause of ischemic stroke or transient ischemic attack (TIA).1 Recently published randomized controlled trials comparing PFO closure versus medical therapy have shown no difference in the risk of recurrent stroke or TIA between the two groups,2,3,4 However, meta-analyses, including non-randomized data or focusing on trials which used the Amplatzer closure device, indicate an evidence of a benefit of PFO closure.5,6 PFO closure appears to be a very appealing concept, potentially avoiding the need for long-term anti-platelet or anticoagulation therapy. However, there is a paucity of data regarding efficacy and safety of PFO closure for prevention of stroke or TIA in Asian populations who are more prone to bleeding as a result of blood thinning therapy.7 This study represents our experience with the Amplatzer PFO Occluder (St. Jude Medical, St. Paul, MN, USA) in Korean patients with PFO and history of prior cryptogenic ischemic stroke or TIA.
This study is a prospective observational study from the Gil Medical Center PFO registry. From October 2010 to August 2014, 184 consecutive patients with stroke/recurrent TIA and PFO documented on transesophageal echocardiography (TEE) and no other identifiable cause of the ischemic event, such as no carotid or intracranial artery stenosis, no atrial fibrillation and no thrombus or atheromatous plaque at aortic arch, were analyzed. Of these patients, those who were aged 18–60 and underwent a PFO closure or medical therapy alone were enrolled in this study. PFO closure was determined according to Heart team's discretion (consist of cardiologist, neurologist and radiologist) based on patient's clinical data, echocardiographic findings and patient's preference. Fifteen patients were excluded due to their age, and 5 patients were lost during the follow-up period (Fig. 1). Finally, 164 patients (men: 126 patients, mean age: 48.1±8.5 years) were eligible for this study. The closure group included 72 patients and 92 patients were included in the medical group (medical therapy only). This study was approved by the Institutional Review Board of the Gil Medical Center.
The closure procedure was performed under general anesthesia. After femoral venous access, the PFO was crossed with a 5 Fr multipurpose catheter. The multipurpose catheter was advanced into the left upper pulmonary vein and exchanged over a 0.035 inch J-tipped stiff guidewire for an 8 Fr or 9 Fr guiding sheath. Procedural anticoagulation was initiated with 5000 unit intravenous heparin. The appropriate device size was selected based on TEE measurements of the distance between the PFO and the aortic root. Once selected, the device was advanced through the sheath to the tip and, subsequently, the sheath and device were pulled back as a unit from the left upper pulmonary vein into the left atrium. While maintaining the position of the device, the sheath was gently pulled back allowing deployment of the left atrial disk. Under TEE guidance, the expanded left atrial disk was then retracted together with the sheath to the atrial septum, and, following verification of septal abutment, the sheath was pulled back, further allowing deployment of the right atrial disk. Upon confirmation of successful positioning by both TEE and fluoroscopy, the device was released. Recommended antiplatelet therapy following the procedure included aspirin 100 mg daily and clopidogrel 75 mg daily for at least 3 months. Follow-up transthoracic echocardiography (TTE) or TEE with agitated saline test was performed between 1 and 3 months after the procedure. Procedural success was defined as successful implantation of device without any procedure-related complication and in-hospital mortality or morbidity.
In the medical group, antithrombotic therapy was left to the discretion of the treating physician and could have included anti-platelet therapy or oral anticoagulation.
Septal hypermobility refers to interatrial septal excursion during the cardiac cycle of 10 mm or more from the midline. Shunt refers to agitated saline contrast (bubbles) appearing in the left atrium within 3 cardiac cycles of right atrial opacification. The degree of shunting was defined as small if 3–9 contrast bubbles appeared, moderate if 10–30 contrast bubbles appeared, and large if more than 30 contrast bubbles appeared in the left atrium.8
Definition of risk of paradoxical embolism (RoPE) score was based on the previously published data.9 The primary end point was a composite of death, ischemic stroke, TIA, or peripheral embolism. Secondary end points were individual components of the primary end point as well as cardiovascular death, new-onset atrial fibrillation, myocardial infarction (MI), hospitalization related to the PFO or its treatment, device problems and bleeding.
Continuous data are expressed as mean±standard deviation and normality tests were performed in each variable for determination of whether or not a data set is well-modeled by a normal distribution. The baseline characteristics of the two groups were compared using the two-sample t-test for continuous variables, and chi-square test and Fisher's exact test for categorical variables. Analysis of longitudinal data for the primary endpoint was performed using Kaplan-Meier estimates with the log-rank test. A p-value of <0.05 was considered significant. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA), version 20.
Among the 164 eligible patients, 72 were assigned to the closure group and 92 to the medical group. Baseline characteristics were similar between the two groups, except age which was higher in the medical group and RoPE scorewhich was higher in the closure group. On TEE, moderate to large right to left shunt were more common in the closure group compared to the medical group (81.9% vs. 63.0%, p=0.009). The presence of shunt at rest or septal hypermobility was also more common in the closure group than in the medical group (61.1% vs. 23.9%, p<0.0001) (Table 1).
Device implantation was attempted in 72 patients in the closure group and was completed in all patients. In most patients, a 5 Fr multipurpose catheter was passed easily through the PFO, but, in some patients, Swartz™ SL sheath (St. Jude Medical, Plymouth, MN, USA) was necessary for strong back-up support for passage of the guidewire through the PFO. In 12 patients, 18 mm devices were implanted, 25 mm devices in 56 patients, and 30 mm devices in 4 patients. One left atrial wall perforation occurred, but did not require additional management. Therefore, implantation was deemed successful in 71 of the 72 patients (98.6%). Between 1 and 3 months after the index procedure, 55 patients (76.4%) in the closure group underwent TTE or TEE with agitated saline test to confirm the residual shunt after PFO closure. Of these, the device was correctly positioned in all patients (47 with no shunt, 5 with minimal shunt, 1 with moderate shunt, and 2 with severe shunt). Effective closure was defined as closure with no or minimal shunting, and therefore, was achieved in 52 of the 55 patients (94.6%). Among the 92 patients in the medical group, all patients had antiplatelet or anticoagulant treatment at discharge, and none crossed over to the closure group during the follow-up period. From 3 months onward, the use of antiplatelet agent was significantly less frequent in the closure group than in the medical group (p=0.005). Use of oral anticoagulation was rare, but similar in both groups (Table 2). The mean duration of follow-up was 22 months in the closure group and 20 months in the medical group.
Primary end points occurred in 2 patients (2.8%) in the closure group and 2 patients (2.2%) in the medical group (p=1.000) (Table 3). Fig. 2 shows the corresponding Kaplan-Meier curves for the primary composite end point. In an analysis of the individual components of the primary end point, TIA occurred in 2 patients (2.0%) in the closure group, and one stroke (1.0%) occurred in the medical group. There was no peripheral embolic event in either group. One patient in the medical group (1.0%) and no patient in the closure group died due to MI at 3 months after enrollment. Otherwise, there was no evidence of device-associated thrombi or new-onset atrial fibrillation in either group. There was also no hospital admission related to PFO or bleeding complications in either group.
In our study group, PFO closure with the Amplatzer PFO occluder for secondary prevention of stroke or TIA did not result in any difference in the risk of embolic events or death, as compared with medical therapy alone. Other findings of the current study are 1) the recurrence rate of embolic events in our patients was very low; only 1.8% during mean 20-month follow-up period, which is lower compared to other similar studies.2,3,4 2) During the follow-up period, the use of antiplatelet agent was lower in the closure group than in the medical group, which might be a crucial point in an Asian population like ours.
Based on randomized data, percutaneous PFO closure in patients with ischemic stroke did not appear to be superior to medical therapy.2,3,4 These results are consistent with those of our study. Possible reasons include heterogeneous population, insufficient sample size, inappropriate patient selection, low event rate, short follow-up duration, or adverse events eliminating a beneficial effect of PFO closure. On the contrary, meta-analyses, including non-randomized data or focusing on trials which used the Amplatzer closure device, indicate a benefit of PFO closure.5,6 Furthermore, some studies have shown that the echocardiographic characteristics of PFO in those with high RoPE scores are strongly associated with recurrent stroke or TIA,10 suggesting that echocardiographic factors seen in high RoPE score patients might provide clues for the PFO-relatedness of the initial stroke in these patients. In our study, higher RoPE score was observed in the closure group. Besides, moderate to large right to left shunt and the shunt at rest or septal hypermobility were more common in the closure group, indicating more benefit obtained from PFO closure. Therefore, its indication should be carefully assessed, and future studies need to focus on optimal patient.
We should also be careful on the fact that the PFO closure procedure is associated with some risks such as bleeding, vascular injury, device embolization, thrombus formation on the device, and tamponade. In most studies, however, the data showed that it can be achieved at a high rate with low complication rates due to the procedure itself.10,11,12 In our study, only one procedure-related complication, which did not require additional management, was noted. Also, there was no death or severe complication related to the procedure. This becomes highly relevant as the medical treatment for ischemic stroke or TIA associated with PFO may include dual antiplatelet and/or anticoagulation therapy with their known potential risks. In our study, the use of antiplatelet agent in the closure group was lower than in the medical group, which might be a crucial point in the Asian population, in which hemorrhagic stroke risk (especially with regard to long-term use of antiplatelet or anticoagulation therapy) tends to be higher than in Western populations.7,13 Currently, there are no studies proving benefit of a specification medication or duration of medication for secondary prevention of ischemic stroke in patients with PFO, and both anti-platelets and/or anticoagulation are considered reasonable options,9,14,15,16 pointing that the risk of bleeding would be increased in a time dependent manner with these medications. Given the safe and effective closure observed with the Amplatzer PFO Occluder, as in our study, PFO closure may be a reasonable approach for patients requiring long-term antiplatelet or anticoagulation therapy. However, device-related complications (erosion, migration, thrombosis) during the follow-up period after the procedure as well as residual shunt all play a role in complications or recurrent events.17,18 Therefore, increasing efforts to optimize device characteristics are necessary.
This study has several limitations as follows. First, this is a non-randomized study with different patient's profile and medications between both groups, and the relatively small sample size and low event rate in a single center study render it difficult to generalize the findings to a larger population. Second, relatively short echocardiographic and clinical follow-up duration in a small population is inadequate for definitive assessment of the absolute risk for recurrent events and bleeding complication of antiplatelet or anticoagulation therapy. Third, selection bias and incomplete follow-up (e.g., shunt grade) make direct comparison of both groups impossible.
Our study comparing PFO closure, using the Amplatzer PFO Occluder and medical therapy alone, in patients with ischemic stroke or recurrent TIA did not show any significant difference in reduction of the risk of recurrent embolic events or death in the real world's setting. However, further investigation of the efficacy and safety of PFO closure is needed in the Asian population, which is prone to bleeding from long-term use of antiplatelet or anticoagulation therapy.
Figures and Tables
Table 1
Baseline Clinical and Transesophageal Echocardiographic Characteristics
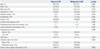
Table 2
Medication during Follow-Up Period

References
1. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988; 318:1148–1152.

2. Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012; 366:991–999.

3. Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013; 368:1083–1091.

4. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013; 368:1092–1100.

5. Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM, Kapadia SR. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv. 2012; 5:777–789.

6. Wolfrum M, Froehlich GM, Knapp G, Casaubon LK, DiNicolantonio JJ, Lansky AJ, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart. 2014; 100:389–395.

7. Ikeda Y, Shimada K, Teramoto T, Uchiyama S, Yamazaki T, Oikawa S, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014; 312:2510–2520.

8. Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001; 345:1740–1746.

9. Kent DM, Thaler DE. RoPE Study Investigators. The risk of paradoxical embolism (RoPE) study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011; 12:185.

10. Thaler DE, Ruthazer R, Weimar C, Mas JL, Serena J, Di Angelantonio E, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs. other PFOs. Neurology. 2014; 83:221–226.

11. Paciaroni M, Agnelli G, Bertolini A, Pezzini A, Padovani A, Caso V, et al. Risk of recurrent cerebrovascular events in patients with cryptogenic stroke or transient ischemic attack and patent foramen ovale: the FORI (Foramen Ovale Registro Italiano) study. Cerebrovasc Dis. 2011; 31:109–116.

12. Mazzucco S, Bovi P, Carletti M, Tomelleri G, Golia G, Stegagno C, et al. A model of multi-disciplinary approach to the diagnosis and treatment of young patients with cryptogenic stroke and patent foramen ovale. Cardiol Young. 2012; 22:327–334.

13. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008; 118:2702–2709.
14. Wahl A, Jüni P, Mono ML, Kalesan B, Praz F, Geister L, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012; 125:803–812.

15. Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:2 Suppl. e601S–e636S.
16. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002; 105:2625–2631.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download