Abstract
Purpose
Tolfenamic acid (TA), a non-steroidal anti-inflammatory drug, is known to exhibit antitumor effects in various cancers apart from nasopharyngeal cancer (NPC). NPC exhibits high invasiveness, as well as metastatic potential, and patients continue to suffer from residual, recurrent, or metastatic disease even after chemoradiation therapy. Therefore, new treatment strategies are needed for NPC. In this study, we investigated the efficacy and molecular mechanisms of TA in NPC treatment.
Materials and Methods
TA-induced cell death was detected by cell viability assay in the NPC cell lines, HNE1 and HONE1. Wound healing assay, invasion assay, and Western blot analysis were used to evaluate the antitumor effects of TA in NPC cell lines.
Results
Treatment with TA suppressed the migration and invasion of HNE1 and HONE1 cells. Hepatocyte growth factor enhanced the proliferation, migration, and invasion abilities of NPC cells. This enhancement was successfully inhibited by TA treatment. Treatment with TA increased phosphorylation of p38, and the inhibition of p38 with SB203580 reversed the cytotoxic, anti-invasive, and anti-migratory effects of TA treatment in NPC cell lines. Moreover, inhibition of p38 also reversed the decrease in expression of Slug that was induced by TA treatment.
Nasopharyngeal cancer (NPC), a malignancy of the head and neck, is rare in most regions worldwide; however; it is a relatively common form of cancer in Southern China, Southeast Asia, the Arctic, and the Middle East/North Africa.12 Even though its complex etiology is not completely understood, ethnicity, environmental factors, and Epstein-Barr virus infection are some of the risk factors associated with NPC.1 NPC is highly responsive to radiotherapy and/or chemotherapy, while surgical extirpation3 of NPC is difficult. Since NPC is highly invasive and metastatic and differs from other head and neck cancers, surgical treatment is only attempted to salvage residual or recurrent disease with limited success rate.45 Further, even though the results of primary curative chemoradiation show good survival, 4–23% of patients still suffer from residual, recurrent, and metastatic disease.36 Therefore, considerable effort is being invested into developing an alternative approach for NPC treatment.
Chemoprevention has recently come into the spotlight in cancer treatment not only for interrupting the carcinogenic process but also for inhibiting the recurrence of lesions.7 Cancer chemoprevention inhibits, delays, or reverses the process of carcinogenesis. The effectiveness of chemopreventive therapy depends on the efficacy, safety, availability, and cost-effectiveness of these drugs.89 Tolfenamic acid (TA) is a popular non-steroidal anti-inflammatory drug (NSAID), which is proven safe and is inexpensive for use in clinical settings, especially for the treatment of migraine. Further, besides its original anti-inflammatory effects, TA shows excellent anti-tumor effects in various cancers, including colorectal, thyroid, prostate, ovarian, lung, breast, and esophageal cancers.8910 However, the effectiveness and possible mechanism of action of TA in treatment of NPC has not been studied. We hypothesized that TA is a promising chemopreventive agent for NPC. In this investigation, we investigated whether TA has anti-proliferative and anti-progression effects on NPC cell lines, especially via p38 mitogen-activated protein kinase (p38) mediated-down-regulation of Slug.
HNE1, HONE1, and HaCaT cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). HNE1 cell line was maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS). HONE1 cell line was maintained in RPMI1640 medium (Gibco) supplemented with 10% FBS. Cells were incubated at 37℃ in an atmosphere containing 5% CO2. TA (Sigma-Aldrich, St. Louis, MO, USA). SB203580 (Calbiochem, La Jolla, CA, USA, No. 559389) [p38 mitogen-activated protein kinase (MAPK) inhibitor] was dissolved according to the manufacturer's instructions for use in in vitro studies.
The viability of the cells after treatment with various concentrations (0, 5, 10, 20, 30, 50, 75, 100, 150, or 200 µM) of TA was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) assay. Briefly, MTT solution was added to 40 mL of cell suspension for 4 h, and the insoluble formazan product was dissolved in 100 µL of dimethylsulfoxide. The optical density of each culture well was measured at 540 nm using a microplate reader (Bio-Tek, Winooski, VT, USA). The results are represented as percentages relative to untreated control cells.
Cell migration was measured using the wound-healing assay as previously described.11 Briefly, cells were grown to confluent monolayers, and then wounded by scratching the surface as uniformly as possible with a 1-mL pipette tip. HNE1 and HONE1 cells were pre-treated with hepatocyte growth factor (HGF) (10 ng/mL), followed by treatment with TA (0, 10, 30, 50, 75, or 100 µM). After 24 h of incubation, cells were washed and stained with 0.02% crystal violet (Sigma-Aldrich). The images of the wound area were captured with an Olympus SC 35 camera (Tokyo, Japan) connected to an inverted microscope. The results are presented as percentage closure area of tumor cell lines compared to the control.
The invasion assay was carried out as previously described.12 Briefly, 24-well Transwell filters with an 8-µm pore size and filters coated with collagen were used. Both HNE1 and HONE1 cells (2×104) in the upper chamber were pretreated with TA (0, 10, 50, or 100 µM) with or without 10 ng/mL HGF. Both inserts and lower wells were treated with vehicle control (dimethyl sulfoxide, DMSO), TA, and HGF. The chambers were incubated for 24 h at 37℃ in an atmosphere containing 5% CO2. After 24 h, the cells in the insert were gently removed using a cotton swab. Cells on the lower surface of the filter were fixed and stained by hematoxylin and eosin staining solutions. The number of invading cells was counted in four representative fields per membrane by using light microscopy at 40× magnification with Image J program (National Institutes of Health, Bethesda, MD, USA). Invasion rate is presented as a percentage compared to control.
After cells were treated with TA (0, 10, 30, or 50 µM), total proteins were extracted using the ProteoExtract® Subcellular Proteome Extraction Kit (Calbiochem), following the manufacturer's instructions. Protein concentrations were measured using the BCA assay (Pierce, Rockford, IL, USA). The proteins were separated by electrophoresis on 12% and 10% sodium dodecyl sulfate polyacrylamide (SDS) gels. An equal amount of protein (10 µg) was loaded in each lane. After electrophoresis, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes. The membrane was blocked in Tris-buffered saline tween-20 (TBST) containing 5% non-fat milk for 1 h, and were then incubated overnight at 4℃ with primary antibodies (1:1000 dilution). All primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). After washing the membrane extensively, incubation with horseradish peroxidase-conjugated secondary antibody (1:1000 dilution, Cell Signaling Technology) was performed for 1 h at room temperature. Protein bands on the blots were visualized using ECL Plus Western Blot detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
As shown in Fig. 1, 24 h and 48 h after treatment with TA, cell viability significantly decreased in both NPC cell lines. We examined the effects of different concentrations of TA on both HNE1 and HONE1 cells and found that TA significantly inhibited NPC cell growth in a dose- and time-dependent manner (Fig. 1A). In terms of general toxicity of TA, cytotoxicity was not caused by TA treatment with a cytotoxic concentration to NPC cells (Fig. 1B).
HGF is a potent enhancer of invasion and progression of various head and neck carcinoma cells. To analyze the suppressive effect of TA on the migration of NPC cell lines, we performed a wound-healing assay. As shown in Fig. 2A, treatment with TA suppressed the migration of HNE1 and HONE1 cells. HGF enhanced the proliferation and migration abilities of the NPC cells, and this enhancement was successfully inhibited by TA treatment (Fig. 2A). We further addressed the effect of TA on invasion of NPC cells using the Transwell invasion assay. Cotreatment of cells with TA and HGF significantly inhibited cell invasion, compared to that observed with treatment with HGF alone, especially in HNE1 cells (Fig. 2B). These results indicated that TA inhibits HGF-induced NPC cell migration and invasion in a dose-dependent manner.
Next, we evaluated TA induced-changes at the protein level in NPC cells. To determine whether MAPK pathway mediated the observed cytotoxic, anti-invasive, and anti-migratory responses upon TA treatment, we determined the phosphorylation levels of p38 and JNK. A representative western blot is shown in Fig. 3A. An increase in phosphorylation of p38 as well as JNK was observed (Fig. 3A) post TA treatment. Since the increase in phosphorylation of p38 signaling was more significant than that of JNK, we decided to focus on the p38-mediated signaling pathways. Treatment with the p38 inhibitor SB203580 for 48 h caused a significant increase in the viability of both NPC cell lines (Fig. 3B).
As is shown in Fig. 4A, TA-induced inhibition of migration in HGF-treated NPC cells was partially reversed upon p38 inhibition. Similarly, attenuation of invasion following TA treatment was also restored upon SB203580 treatment (Fig. 4B).
The activation of Slug, which is a member of the Snail superfamily and plays pivotal role in migration and invasion by inducing epithelial-mesenchymal transition (EMT),13141516 was evaluated by western blot analysis. TA treatment decreased the phosphorylation of Slug, and p38 inhibition reversed the effect of TA in both NPC cell lines (Fig. 5). These results suggest that anti-tumor effects of TA in NPC are a result of p38 MAPK mediated-down-regulation of Slug.
Despite the high response rate of NPC to radiotherapy and chemotherapy, a considerable number of patients eventually relapse.17 As an alternative, various biologic chemotherapeutics have been developed that target pathways mediating cell proliferation, angiogenesis, migration, and invasion.1819 However, these are not effective molecular targets against NPC, and the life expectancy of patients with recurrent or metastatic NPC remains low. Several studies combining biologic chemotherapeutics against specific molecular targets with conventional chemotherapeutics have shown a median overall survival of only 8–12 months; therefore, the value of adding biologic agents remains unclear.2021 Thus, development of new treatment strategies for NPC is needed.
Chemoprevention is an unexplored treatment modality in the field of cancer research. Cancer chemoprevention aims to delay, prevent, or reverse the development of cancers, by longterm intake of a natural or synthetic biological agent.7 A potential target of chemoprevention is down-regulation of chronic inflammatory responses, which may contribute to the prevention of cancer initiation.7 Several preclinical and epidemiological investigations have shown strong evidence that NSAIDs reduce the overall risk of developing various cancers.10 To develop strategies for treatment of NPC, understanding the mechanisms of action of NSAIDs, rather than depending on historical epidemiological observations, is needed.
In this study, we investigated the efficacy of TA in NPC treatment and the molecular mechanism of TA in inhibition of NPC. TA-induced cell death was detected by cell viability assay in the NPC cell lines HNE1 and HONE1. TA successfully inhibited HGF-induced migration and invasion of NPC cell lines. TA mediated its effects on NPC cell lines via the p38 MAPK and JNK pathways. We observed increased phosphorylation of p38, and the inhibition of p38 with SB203580 reversed the cytotoxic, anti-invasive, and anti-migratory effects of TA treatment in NPC cell lines. Moreover, inhibition of p38 also reversed a TA treatment-induced decrease in Slug. This suggests that TA-induced anti-tumor effects in NPC cell lines might be attributed to a p38-induced down-regulation of Slug, while inhibition of p38 could reverse these effects.
Activation of p38 MAPK by various external stimuli plays an important role in biological responses, such as inflammation, cell proliferation, regulation of apoptosis, and survival.222324252627 p38 MAPK is involved in tumor growth and progression in many types of human cancers, including colorectal, lung, breast, and thyroid carcinomas,2829 as well as head and neck squamous cell carcinomas.3031 Further, p38 MAPK signaling is associated with sensitivity to chemotherapeutics.27 Activation of non-steroidal anti-inflammatory drug activated gene-1 (NAG-1) via p38 MAPK pathway is responsible for rottlerin-induced apoptosis in the HT29 colon carcinoma cell line.32 Our group previously reported that TA induces apoptosis and growth inhibition via NAG-1 expression in head and neck, as well as anaplastic thyroid, cancers.89 In this study, TA treatment-induced activation of p38, while inhibition of p38 attenuated the effect of TA on cytotoxicity, as well as inhibition of invasion and migration, in NPC cell lines.
Slug is a member of the Snail superfamily, which is a family of zinc-finger transcription factors involved in the pathogenesis of EMT. Elevated expression of Snail enhances cell invasion and migration by down-regulating epithelial markers and up-regulating mesenchymal markers. Expression of Slug is closely associated with tumor recurrence, metastasis, and poor survival in various types of cancers.33343536 Various pathways regulate Slug, such as receptor tyrosine kinases activated by HGF, FGF, or EGF or the RAS-MAPK or PI3K-Akt pathway.3738 HGF is also strongly involved in transcriptional regulation of Snail. HGF-mediated MAPK activation enhances the expression of Snail.39 Inhibition of p38 MAPK is also reported to reverse EMT in advanced tumor phenotype. In the present study, we observed that inhibition of p38 with SB203580 reversed a TA treatmentinduced decrease in Slug. Consequently, re-expression of Slug upon p38 inhibition led to the recovery of cell viability, invasiveness, and migratory abilities in NPC cell lines.
In conclusion, these data demonstrate that TA induces p38-mediated cell death and inhibition of invasion and migration in NPC cells. The TA-induced p38 activation leads to down-regulation of Slug. Further, p38 inhibition with SB203580 reversed the anti-tumor effects of TA treatment in NPC cell lines. These findings suggest that activation of p38 plays a role in mediating TA-induced cytotoxicity, as well as inhibition of invasion and migration, via down-regulation of Slug.
Figures and Tables
Fig. 1
Effects of tolfenamic acid on proliferation of nasopharyngeal cancer cell lines and a normal keratinocyte cell line. Cells were seeded in 96-well tissue-culture plates, at a density of 2×103 cells/well in a final volume of 100 µL of medium, and were allowed to attach for 24 h. The cells were then treated with ten different concentrations of tolfenamic acid (TA) for 24 h and 48 h. Cell proliferation was estimated by the MTT assay. (A) Nasopharyngeal cancer cell lines, (B) HaCaT cell lines. Values are represented as mean±SD from three independent experiments. *p<0.05 and ***p<0.001 compared to the control group. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.

Fig. 2
Effect of tolfenamic acid (TA) on the migratory and invasive abilities of nasopharyngeal cancer cells. The migration capability after HGF/(TA) treatment was investigated using wound healing assay. (A) HNE1 and HONE1 cells were either treated with 10 ng/mL HGF along with TA (0, 10, 30, 50, 75, 100 µM) or left untreated. Confluent monolayers of HNE1 were wounded by scratching the surface as uniformly as possible with a 1-mL pipette tip. TA treatment significantly inhibited HGF-induced enhancement of cell migration in a dose-dependent manner. (B) HNE1 and HONE1 cells (2×104) in the upper chamber were pretreated with TA (0, 10, 30, 50, 75, 100 µM) and then either treated with 10 ng/mL HGF or left untreated. After 24 h, the number of invading cells was counted in four representative fields per membrane. Co-treatment with TA and HGF significantly inhibited cell invasion compared to treatment with HGF alone. The data represent the mean±SD of three independent experiments. *p<0.05, **p<0.01, ***p<0.001. HGF, hepatocyte growth factor.
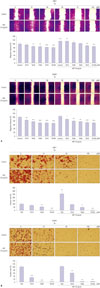
Fig. 3
Effect of tolfenamic acid (TA) on the MAPK pathway in nasopharyngeal cancer cells. (A) Immunoblot of TA-treated cells stained with antibodies against T-p38, p-p38, T-JNK, and p-JNK. Western blot analysis showed that TA-enhanced phosphorylation of p38 and JNK in HNE1 and HONE1 cells. (B) HNE1 and HONE1 cells were treated with HGF (10 ng/mL), SB203580 (0.5 µM), and TA (50 µM) alone or in combination, as indicated. Cell viability assay was performed to investigate the cytotoxic effect of TA in nasopharyngeal cancer cells in the absence or presence of SB203580. The data represent the mean±SD of three independent experiments. *p<0.05, ***p<0.001 by one-way ANOVA. MAPK, mitogen-activated protein kinase.
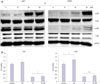
Fig. 4
Effect of a p38 inhibitor on anti-migratory and anti-invasive abilities of tolfenamic acid (TA) in nasopharyngeal cancer cells. HNE1 and HONE1 cells were treated with HGF (10 ng/mL), SB203580 (0.5 µM), and TA (50 µM) alone or in combination, as indicated. (A) Wound healing and (B) invasion assays were used to determine the anti-migratory and anti-invasive abilities of TA in nasopharyngeal cancer cells in the absence or presence of SB203580. The data represent the mean±SD of three independent experiments. *p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA. DMSO, dimethyl sulfoxide; HGF, hepatocyte growth factor.
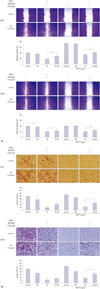
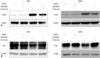
Fig. 5
Effect of p38 inhibition on Slug expression in tolfenamic acid (TA)-treated nasopharyngeal cancer cells. HNE1 and HONE1 cells were treated with TA (50 µM) and SB203580 (0.5 µM) alone or in combination, as indicated. Immunoblot of treated cells stained with antibodies against (A) p-p38 (30 min) and (B) Slug (3 h). Western blot analysis showed that TA reduced phosphorylation of p38 and Slug, while this effect was reversed by SB203580 treatment. DMSO, dimethyl sulfoxide.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program (2012M3A9B2052870) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2015R1A2A1A01002968).
References
1. Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014; 80:384–392.

2. Li K, Lin GZ, Shen JC, Zhou Q. Time trends of nasopharyngeal carcinoma in urban Guangzhou over a 12-year period (2000-2011): declines in both incidence and mortality. Asian Pac J Cancer Prev. 2014; 15:9899–9903.

3. Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005; 61:1107–1116.

4. Na'ara S, Amit M, Billan S, Cohen JT, Gil Z. Outcome of patients undergoing salvage surgery for recurrent nasopharyngeal carcinoma: a meta-analysis. Ann Surg Oncol. 2014; 21:3056–3062.
5. Chan JY, Tsang RK, Wei WI. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015; 37:487–492.

6. Li JX, Huang SM, Jiang XH, Ouyang B, Han F, Liu S, et al. Local failure patterns for patients with nasopharyngeal carcinoma after intensity-modulated radiotherapy. Radiat Oncol. 2014; 9:87.

7. Steward WP, Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013; 109:1–7.

8. Chang JW, Kang SU, Choi JW, Shin YS, Baek SJ, Lee SH, et al. Tolfenamic acid induces apoptosis and growth inhibition in anaplastic thyroid cancer: involvement of nonsteroidal anti-inflammatory drug-activated gene-1 expression and intracellular reactive oxygen species generation. Free Radic Biol Med. 2014; 67:115–130.

9. Kang SU, Shin YS, Hwang HS, Baek SJ, Lee SH, Kim CH. Tolfenamic acid induces apoptosis and growth inhibition in head and neck cancer: involvement of NAG-1 expression. PLoS One. 2012; 7:e34988.

10. Jeong JB, Choi J, Baek SJ, Lee SH. Reactive oxygen species mediate tolfenamic acid-induced apoptosis in human colorectal cancer cells. Arch Biochem Biophys. 2013; 537:168–175.

11. Shin HA, Shin YS, Kang SU, Kim JH, Oh YT, Park KH, et al. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J Radiat Res. 2014; 55:32–40.

12. Lee BS, Kang S, Kim KA, Song YJ, Cheong KH, Cha HY, et al. Met degradation by SAIT301, a Met monoclonal antibody, reduces the invasion and migration of nasopharyngeal cancer cells via inhibition of EGR-1 expression. Cell Death Dis. 2014; 5:e1159.

13. Choi MJ, Cho KH, Lee S, Bae YJ, Jeong KJ, Rha SY, et al. hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene. 2015; 34:3402–3412.

14. Zhao X, Sun B, Sun D, Liu T, Che N, Gu Q, et al. Slug promotes hepatocellular cancer cell progression by increasing sox2 and nanog expression. Oncol Rep. 2015; 33:149–156.

15. Merikallio H, T TT, Pääkkö P, Mäkitaro R, Kaarteenaho R, Lehtonen S, et al. Slug is associated with poor survival in squamous cell carcinoma of the lung. Int J Clin Exp Pathol. 2014; 7:5846–5854.
16. Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, et al. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013; 7:322–330.
17. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006; 15:1765–1777.

18. Tsang J, Lee VH, Kwong DL. Novel therapy for nasopharyngeal carcinoma--where are we. Oral Oncol. 2014; 50:798–801.
19. Chiang AK, Mak NK, Ng WT. Translational research in nasopharyngeal carcinoma. Oral Oncol. 2014; 50:345–352.

20. You B, Le Tourneau C, Chen EX, Wang L, Jarvi A, Bharadwaj RR, et al. A Phase II trial of erlotinib as maintenance treatment after gemcitabine plus platinum-based chemotherapy in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Am J Clin Oncol. 2012; 35:255–260.

21. Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005; 23:3568–3576.

22. Kim HS, Lee JH, Park HS, Lee GS, Kim HW, Ha KT, et al. Schizandra chinensis extracts induce apoptosis in human gastric cancer cells via JNK/p38 MAPK activation and the ROS-mediated/mitochondria-dependent pathway. Pharm Biol. 2015; 53:212–219.

23. Zhao B, Li X. Altholactone induces reactive oxygen species-mediated apoptosis in bladder cancer T24 cells through mitochondrial dysfunction, MAPK-p38 activation and Akt suppression. Oncol Rep. 2014; 31:2769–2775.

24. Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, et al. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014; 67:193–200.

25. Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009; 9:537–549.

26. Chiacchiera F, Simone C. Signal-dependent regulation of gene expression as a target for cancer treatment: inhibiting p38alpha in colorectal tumors. Cancer Lett. 2008; 265:16–26.

27. Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014; 344:174–179.

28. Matrone A, Grossi V, Chiacchiera F, Fina E, Cappellari M, Caringella AM, et al. p38alpha is required for ovarian cancer cell metabolism and survival. Int J Gynecol Cancer. 2010; 20:203–211.
29. Grossi V, Simone C. Special Agents Hunting Down Women Silent Killer: The Emerging Role of the p38α Kinase. J Oncol. 2012; 2012:382159.
30. Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci. 2002; 115(Pt 15):3193–3206.

31. Hsieh YH, Wu TT, Huang CY, Hsieh YS, Hwang JM, Liu JY. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007; 67:4320–4327.

32. Lim JH, Woo SM, Min KJ, Park EJ, Jang JH, Seo BR, et al. Rottlerin induces apoptosis of HT29 colon carcinoma cells through NAG-1 upregulation via an ERK and p38 MAPK-dependent and PKC δ-independent mechanism. Chem Biol Interact. 2012; 197:1–7.

33. Sharma-Walia N, Patel K, Chandran K, Marginean A, Bottero V, Kerur N, et al. COX-2/PGE2: molecular ambassadors of Kaposi's sarcoma-associated herpes virus oncoprotein-v-FLIP. Oncogenesis. 2012; 1:e5.

34. Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000; 257:1–12.

35. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007; 7:415–428.

36. Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005; 8:197–209.

37. Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001; 1:37–49.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download