Abstract
Purpose
When performing coronary angiography (CAG), diagnostic catheter intubation to the ostium can cause damping of the pressure tracing. The aim of this study was to determine the predictors of atherosclerotic ostial stenosis in patients showing pressure damping during CAG.
Materials and Methods
In total, 2926 patients who underwent diagnostic CAG were screened in this study. Pressure damping was defined as an abrupt decline of the coronary blood pressure with a blunted pulse pressure after engagement of the diagnostic catheter. According to CAG and intravascular ultrasound (IVUS), we divided damped ostia into two groups: atherosclerotic ostial lesion group (true lesion group) and non-atherosclerotic ostium group (false lesion group). Clinical and angiographic characteristics were compared between the two groups.
Results
The overall incidence of pressure damping was 2.3% (68 patients and 76 ostia). Among the pressure damped ostia, 40.8% (31 of 76 ostia) were true atherosclerotic ostial lesions (true lesion group). The true lesion group had more frequent left main ostial damping and more percutaneous coronary interventions (PCIs) performed on non-ostial lesions, compared to the false lesion group. On multivariate logistic regression analysis, left main ostial damping [hazard ratio (HR) 4.11, 95% confidence interval (CI) 1.24-13.67, p=0.021] and PCI on non-ostial lesion (HR 5.34, 95% CI 1.34-21.27, p=0.018) emerged as independent predictors for true atherosclerotic ostial lesions in patients with pressure damping.
During coronary angiography (CAG), diagnostic catheter intubation of the ostium often leads to a damping of the pressure tracing. Pressure damping can occur when the outer diameter of the intubated catheter is bigger than the ostial diameter, or the tip of the catheter has been intubated too deep pass the ostium and is against the vessel wall. Although catheter induced coronary spasm has been suggested as a common reversible mechanism of damping,1 atherosclerotic ostial stenosis is an important differential diagnosis. Ostial stenosis is an unusual manifestation of coronary atherosclerosis and recognizing it is important, not only because there is poor prognosis with left main stenosis, but also because of the inherent risks that can occur during catheterization when the left coronary ostium is involved.123 Regardless, symptomatic atherosclerotic ostial stenosis, whether right or left, requires revascularization therapy. To investigate true atherosclerotic lesions at the coronary ostia, intravascular imaging modality can be used, in addition to diagnostic CAG. Although previous studies have reported on the clinical utility of intravascular ultrasound (IVUS) for coronary ostial disease,456 minimal data is available on the predictors of atherosclerotic ostial stenosis in patients showing pressure damping during diagnostic CAG. This retrospective study was conducted to investigate the overall incidence of pressure damping and to determine the clinical or angiographical predictors of true atherosclerotic ostial lesions in patients with pressure damping during diagnostic CAG.
Consecutive patients who underwent diagnostic CAG at Ulsan University Hospital between September 2010 and March 2012 were included in this study. A diagnostic CAG was performed for suspicious cases of myocardial ischemia based on the clinical presentation and positive findings from non-invasive tests. We retrospectively reviewed angiographic records for ostial pressure damping after the operator injected 1-3 doses of 200 µg intracoronary nitroglycerin and a cusp injection with the catheter positioned just below the coronary ostia or a continuous injection of contrast while withdrawing the catheter (for exclusion of catheter induced coronary spasm) and after catheter maneuvering (for exclusion of mal-alignment of the catheter, such as against the vessel wall). We used a diagnostic Judkins 5 F or 6 F catheter without a side hole via either a radial or femoral artery approach at the operator's discretion. Pressure damping was defined as an abrupt decline in coronary blood pressure with blunted pulse pressure after engagement of the diagnostic catheter tip (Fig. 1).7 We differentiated between a damped waveform (in which both systolic and diastolic pressure were reduced) and a ventricularized waveform (in which systolic pressure was preserved but diastolic pressure was reduced) and ruled out ventricularized waveforms. The exclusion criteria were catheter induced coronary spasm, hyperthyroidism, congenital vascular malformations, history of radiation exposure, vasculitis and past history of aortic valve replacement. This study was approved by the Ulsan University Hospital Institutional Review Board (IRB) ethics committee.
According to CAG and IVUS, subjects were divided into two groups: true atherosclerotic ostial lesion group (true lesion group) and non-atherosclerotic ostium group (false lesion group). The true atherosclerotic ostial lesions of the left (left main) or right coronary artery (RCA) were defined as a luminal narrowing of ≥50%, located within 3 mm of the aorta on the least foreshortened angiographic projection8 or ostial plaque burden of ≥40% via off-line IVUS analysis. IVUS was done all patients in the two groups. Negative remodeling was defined as a remodeling ratio of <0.95. Remodeling ratio was defined as the ratio of the external elastic membrane (EEM) at the lesion site to the EEM at the distal reference site.9 Eccentricity was defined as the ratio of maximal plaque and media diameter to the minimal plaque and media diameter at maximal plaque burden site. Clinical and angiographic comparisons were made between the two groups and gray scale IVUS imaging was analyzed. Percutaneous coronary intervention (PCI) at ostial lesions and non-ostial lesions was performed according to the operator's discretion, taking into account the clinical significance of the lesion.
Quantitative coronary angiographic analysis (CAAS 5.10, Pie Medical Imaging B.V., Maastricht, the Netherlands) was done using automated edge-detection algorithms. IVUS was performed using motorized transducer pullback (0.5 mm/s) and a commercial scanner (Eagle Eye, Volcano Corporation, Rancho Cordova, CA, USA) consisting of a rotating 20-MHz transducer within a 2.9 F imaging sheath. Using computerized planimetry, off-line IVUS analysis was performed by an investigator blinded to the clinical characteristics of the subjects.
Results are presented as mean±standard deviation (SD) for continuous variables and frequency (percentages) for categorical variables. We compared demographic characteristics and variables between the true lesion and false lesion groups using Student's t-test and the Mann-Whitney U test for continuous variables and the Pearson χ2 test and the Fisher's exact test for categorical variables. Multivariate logistic regression analysis was performed to assess the independent predictors of coronary atherosclerotic ostial stenosis after adjusting for age, gender, co-morbidities, smoking status, family history of coronary artery disease (CAD), clinical diagnosis, and all angiographic findings. Statistical significance was defined as p value of <0.05 with a two-tailed test. Statistical analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA).
Among the 2926 consecutive patients who underwent diagnostic CAG, 68 patients were included in the study, and pressure damping was observed in 76 ostia (2.3%). The overall incidence of atherosclerotic coronary ostial lesions with pressure damping was 40.8% (31 of 76 ostia). Baseline characteristics and coronary angiographic data of the cohort are summarized in Table 1. Patients in the true lesion group had more diabetes mellitus and a family history of CAD, compared to the false lesion group. On angiographic findings, the number of diseased vessels was related to the presence of an atherosclerotic ostial lesion (0.7±1.0 in false lesion group vs. 1.5±1.2 in true lesion group, p=0.003). Pressure damping of the left main ostium was more common in the true lesion group than the false lesion group [n=16 (51.6%) vs. n=11 (24.4%), p=0.015]. The frequency of coronary spasm at non-ostial lesions was higher in the false lesion group than the true lesion group (75.6% vs. 16.1%, p<0.001). Catheter change was more frequent in the true lesion group than the false lesion group (80.6% vs. 42.2%, p=0.001). PCI on damped ostium, based on the operator's discretion, was performed more frequently in the true lesion group than the false lesion group {n=14 (45.2%) [left main (n=9), right coronary ostium (n=5)] vs. n=0 (0.0%), p<0.001}. There were significantly more PCIs performed on non-ostial lesions in the true lesion group than in the false lesion group [n=21 (67.7%) vs. n=13 (28.9%), p=0.001].
On multivariate logistic regression analysis, age [hazard ratio (HR) 0.95, 95% confidence interval (CI) 0.90-1.00, p=0.048], left main ostial damping (HR 4.11, 95% CI 1.24-13.67, p=0.021), and PCI on non-ostial lesions (HR 5.34, 95% CI 1.34-21.27, p=0.018) emerged as independent predictors for true atherosclerotic ostial lesion in patients with a damping of pressure tracing (Table 2). No other clinical variables predicted atherosclerotic ostial lesions with pressure damping.
All patients underwent IVUS for confirmation of clinical significant lesions of the damped ostium. The true lesion group showed prominent plaque burden, compared to the false lesion group, on IVUS analysis (Table 3). The true lesion group showed negative remodeling in 10 ostia (32%), while the false lesion group showed the more frequent negative remodeling in 23 ostia (51%) (p=0.035). During the follow-up period of 13.2±22.8 months, there were no deaths or re-hospitalizations observed in either group.
The main findings of this study were as follows: 1) during diagnostic CAG, the overall incidence of pressure damping was 2.3%; 2) among pressure damping ostia, the incidence of true atherosclerotic ostial lesions was 40.8%; and 3) left main ostial damping and PCI on non-ostial atherosclerotic lesions emerged as important predictors for the presence of true atherosclerotic ostial lesions with pressure damping.
An abnormal pressure damping tracing may suggest the presence of an ostial stenosis or spasm, selective engagement of the conus branch, or deep intubation of the coronary artery during CAG.7 Although ostial stenosis is often not recognized clinically, clinical symptoms associated with an abrupt fall in the catheter tip pressure during CAG include dyspnea and chest pain. In particular, significant left main CAD places a large area of myocardium in jeopardy, thereby placing the patient at high risk.10 Although previous studies have reported the clinical utility of IVUS for coronary ostial disease,456 limited data is available on the prediction of a true atherosclerotic ostial lesion in patients showing pressure damping using only diagnostic CAG. Angiographic parameters to predict true atherosclerotic ostial lesions are important, because IVUS is not always easily available in all catheterization laboratories globally.
In our study, results representing real world clinical practice suggested that coronary ostial pressure damping, even after nitroglycerin intracoronary injection and a cusp injection or a continuous catheter-withdrawing injection (for exclusion of catheter induced spasm) or catheter maneuvering (for exclusion of wrong alignment of the catheter), is not rare during CAG (incidence, 2.3%). Additionally, true atherosclerotic ostial lesions among damped ostia were frequent (incidence of 40.8%). Even if other causes of pressure damping, such as catheter induced spasm and wrong alignment of the catheter were ruled out, 59.2% of non-atherosclerotic ostial pressure damping were due to negative remodeling (using IVUS data) and smaller vessel size, compared to the catheter used. In this context, a smaller diagnostic catheter such as a 4 Fr catheter may be useful for excluding non-atherosclerotic ostial pressure damping.
Previous reports have documented frequent arteriosclerosis of the aortic sinuses of Valsalva.11 Mild to moderate arteriosclerosis of the sinuses has been found from autopsies of hearts of soldiers dying on the field and more severe involvement has been seen in older autopsied patients, a mean of age 70. One may assume that atheromatous plaque of the wall of the aortic root results in ostial stenosis of the coronary artery, which is located at the upper part of the sinus of Valsalva.12
In the present study, PCI on non-ostial atherosclerotic lesions emerged as an important predictor for coronary atherosclerotic ostial stenosis. In the same context, the true lesion group showed more numbers of diseased vessels and much severe diameter stenosis, compared to the false lesion group (Table 2). This finding also supports the concept discussed above in regards to the development of the atheromatous plaque. The close relationship between arteriosclerosis and serum cholesterol level has been described in many epidemiologic studies.131415 In the present study, according to IVUS analysis, we confirmed that the true lesion group showed a prominent plaque burden, compared to the false lesion group. These findings also provide evidence of a link between significant ostial stenosis and arteriosclerosis. On the contrary, because the most frequent cause of non-atherosclerotic ostial lesion was negative remodeling among damped ostia when there were no non-ostial atherosclerotic lesions, pressure damped ostium should be carefully evaluated using an additional method such as IVUS.
Left ostial damping was another predictor of coronary atherosclerotic ostial stenosis. There was a significant difference in the percentage of left ostial damping in the two groups (24.4% in false lesion group vs. 51.6% in true lesion group, p=0.015). This finding is consistent with the general concept of the frequent location of RCA and iatrogenic spasm.1617 Previous reports have suggested that spasm is most commonly seen with the right coronary catheter when the catheter tip enters the RCA at an angle producing proximal vessel tenting. The false lesion group had frequent coronary spasm at non-ostial lesions, compared to the true lesion group [n=34 (75.6%) vs. n=5 (16.1%), p<0.001]. In other words, if coronary spasm developed at another non-ostial lesion, there was a high probability of non-atherosclerotic ostial lesion with pressure damping. This finding is consistent with previous reports that multiple sites of spasm may be a clue to the important diagnosis of spasm.181920
There were limitations in this study. This was a retrospective, single-center study. Because the study consisted of small patient numbers, it may have affected the results. Also, the damped ostia were classified into two groups depending on the presence of atherosclerotic stenosis. We, therefore, are unable to know the existence of other ischemia-inducing stenosis. However, we have good reasons to believe that our results are reliable. Firstly, the overall incidence of pressure damped ostia was similar to previous reports. Secondly, the operators actively tried to evaluate true atherosclerotic lesions with pressure damped ostia using IVUS. Nonetheless, a further study with larger patient numbers will be helpful to confirm our findings.
In conclusion, after excluding catheter induced spasm and catheter mal-alignment, true atherosclerotic ostial lesions were found to be common (40.8%) among pressure damped ostia during CAG. Left main ostial damping and the presence of a non-ostial atherosclerotic lesion may suggest a significant true atherosclerotic lesion in the coronary ostium. On the contrary, a pressure damped ostium in the absence of non-ostial atherosclerotic lesions may require additional imaging of the coronary ostium, such as with IVUS, to rule out negative remodeling.
Figures and Tables
Fig. 1
Coronary pressure showed an abrupt decline with a blunted pulse pressure during coronary pressure monitoring, in which both systolic and diastolic pressure were reduced as the catheter occluded the ostium.
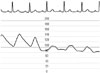
Table 1
Baseline Clinical Characteristics and Coronary Angiographic Data

Table 2
Predictors of True Atherosclerotic Ostial Lesion by Multivariate Logistic Regression Analysis

Table 3
IVUS Findings between the False Lesion Group and True Lesion Group

References
1. Zampieri P, Colombo A, Almagor Y, Maiello L, Finci L. Results of coronary stenting of ostial lesions. Am J Cardiol. 1994; 73:901–903.

2. Barner HB, Naunheim KS, Kanter KR, Fiore AC, McBride LR, Pennington DG, et al. Coronary ostial stenosis. Eur J Cardiothorac Surg. 1988; 2:106–112.

4. Maehara A, Mintz GS, Castagna MT, Pichard AD, Satler LF, Waksman R, et al. Intravascular ultrasound assessment of the stenoses location and morphology in the left main coronary artery in relation to anatomic left main length. Am J Cardiol. 2001; 88:1–4.

5. Abizaid AS, Mintz GS, Abizaid A, Mehran R, Lansky AJ, Pichard AD, et al. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J Am Coll Cardiol. 1999; 34:707–715.

6. Kim SW, Mintz GS, Ohlmann P, Hassani SE, Michalek A, Escolar E, et al. Comparative intravascular ultrasound analysis of ostial disease in the left main versus the right coronary artery. J Invasive Cardiol. 2007; 19:377–380.
7. Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's heart disease: a textbook of cardiovascular medicine. 9th ed. Philadelphia: Elsevier Saunders;2011.
8. Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, et al. Intravascular ultrasound assessment of drug-eluting stent coverage of the coronary ostium and effect on outcomes. Am J Cardiol. 2013; 111:1401–1407.

9. Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001; 37:1478–1492.

10. Dvir D, Assali A, Lev EI, Ben-Dor I, Battler A, Kornowski R. Percutaneous interventions in unprotected left main lesions: novel three-dimensional imaging and quantitative analysis before and after intervention. Cardiovasc Revasc Med. 2010; 11:236–240.

11. Enos WF, Beyer JC, Holmes RH. Arteriosclerosis of the aortic sinuses. Am J Clin Pathol. 1963; 39:506.

12. Beppu S, Minura Y, Sakakibara H, Nagata S, Park YD, Nambu S, et al. Supravalvular aortic stenosis and coronary ostial stenosis in familial hypercholesterolemia: two-dimensional echocardiographic assessment. Circulation. 1983; 67:878–884.

13. Yamazaki T, Nohara R, Daida H, Hata M, Kaku K, Kawamori R, et al. Intensive lipid-lowering therapy for slowing progression as well as inducing regression of atherosclerosis in Japanese patients: subanalysis of the JART study. Int Heart J. 2013; 54:33–39.

14. Nohara R, Daida H, Hata M, Kaku K, Kawamori R, Kishimoto J, et al. Effect of intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness in Japanese patients: Justification for Atherosclerosis Regression Treatment (JART) study. Circ J. 2012; 76:221–229.

15. Cholesterol Treatment Trialists' (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–1681.

16. Friedman AC, Spindola-Franco H, Nivatpumin T. Coronary spasm: Prinzmetal's variant angina vs. catheter-induced spasm; refractory spasm vs. fixed stenosis. AJR Am J Roentgenol. 1979; 132:897–904.

17. Deckelbaum L, Isner JM, Konstam MA, Salem DN. Catheter-induced versus spontaneous spasm. Do these coronary bedfellows deserve to be estranged? Am J Med. 1985; 79:1–4.

18. Persin GA, Matthai WH Jr. Catheter-induced spasm of the left main coronary artery. J Invasive Cardiol. 2000; 12:158–161.
19. MacAlpin RN. Relation of coronary arterial spasm to sites of organic stenosis. Am J Cardiol. 1980; 46:143–153.

20. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014; 129:1723–1730.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download