Abstract
Purpose
Periostin mediates critical steps in gastric cancer and is involved in various signaling pathways. However, the roles of periostin in promoting gastric cancer metastasis are not clear. The aim of this study was to investigate the relevance between periostin expression and gastric cancer progression and the role of stress-related hormones in the regulation of cancer development and progression.
Materials and Methods
Normal, cancerous and metastatic gastric tissues were collected from patients diagnosed with advanced gastric cancer. The in vivo expression of periostin was evaluated by in situ hybridization and immunofluorescent staining. Meanwhile, human gastric adenocarcinoma cell lines MKN-45 and BGC-803 were used to detect the in vitro expression of periostin by using quantitative real-time polymerase chain reaction (PCR) and western blotting.
Results
Periostin is expressed in the stroma of the primary gastric tumors and metastases, but not in normal gastric tissue. In addition, we observed that periostin is located mainly in pericryptal fibroblasts, but not in the tumor cells, and strongly correlated to the expression of α-smooth muscle actin (SMA). Furthermore, the distribution patterns of periostin were broader as the clinical staging of tumors progressed. We also identified a role of stress-related signaling in promoting cancer development and progression, and found that isoprenaline upregulated expression levels of periostin in gastric cancer cells.
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide.1 Extracellular matrix (ECM) proteins play an important role in tumor progression and metastasis.23 Therefore, a better understanding of ECM proteins present in the tumor microenvironment and their influence on cell-ECM interactions could aid the prevention and treatment of gastric cancer progression and metastasis.
The ECM protein periostin has pathophysiological roles in bone formation,4 wound repair,5 vascular diseases,6 and tumor development,7 and has also been implicated in normal physiological
processes, including cardiac development. It is highly expressed in gastric cancer and is indispensable for successful progression and metastasis.89 Clinical studies have showed that high periostin expression correlates with tumor metastasis and poor prognosis.1011 Periostin is of increasing interest in gastric cancer because it is functionally involved in multiple steps of cancer progression and participates in different signaling pathways. These include metastatic niche formation,12 maintenance of stemness,13 EMT,14 angiogenesis, and the survival of tumor cells,15 all of which are indispensable for gastric cancer progression and metastasis. Periostin also participates in and promotes tumor progression through the FAK/Src, Wnt, and PI-3K/AKT signaling pathways.1617 Therefore, periostin represents a promising candidate for the inhibition of gastric cancer progression and metastasis.
In this study, we investigated the expression of periostin in primary gastric tumors and metastases. First, we examined the expression of periostin in the primary tumours and metastases by in situ hybridization. Next, we evaluated the location of periostin and characteristics of its distribution in gastric cancer using immunofluorescence. Then we investigated the effects of isoprenaline on the expression levels of periostin in gastric cancer cells. Our results demonstrated that periostin is overexpressed by pericryptal fibroblasts in gastric cancer tissues, and were positively correlated with the expression of EMT-associated protein, α-smooth muscle actin (SMA). Finally, we found that the distribution patterns of periostin were broader while the clinical staging of tumor progressed, and isoprenaline upregulated expression levels of periostin in gastric cancer cells.
Gastric tissues were collected from the General Hospital of PLA who underwent curative surgical resection with informed consent of patients and the institutional approval. Normal, cancer and metastatic gastric tissue from lymph nodes and tissue adjacent to the tumor were collected from patients diagnosed with gastric cancer. Human gastric adenocarcinoma cell lines MKN-45 and BGC-803 were obtained from the Cancer Institute, Chinese Academy of Medical Science. RPMI-1640 medium, 0.25% trypsin, 0.02% EDTA and fetal bovine serum (FBS) were purchased from Gibco (San Diego, CA, USA); QPCR master mix and the M-MLV reverse transcription system were purchased from Promega (Madison, WI, USA); anti-α-SMA mouse monoclonal antibody and anti-periostin rabbit monoclonal antibody, anti-β-actin mouse monoclonal antibody were purchased from Abcam (Boston, MA, USA); peroxidase-conjugated affinipure goat anti-rabbit IgG, peroxidase-conjugated affinipure goat anti-mouse IgG, Alexa Fluor594-conjugated affinipure goat anti-rabbit IgG and Alexa Fluor488-conjugated affinipure goat anti-mouse IgG were purchased from Cell Signaling Technology (Boston, MA, USA).
Antisense and sense cRNA probes were prepared by in vitro transcription. An EcoRI-XbaI fragment of human and mouse periostin cDNA fragment were labeled by digoxygenin using a DIG RNA Labeling Kit (Roche Applied Science, Indianapolis, IN, USA).18 In situ hybridization was performed manually on paraffin-embedded sections (5 mm thick) as described previously.19 The signals were developed using nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indolylphosphate.
Immunohistochemistry was performed as previously described.20 Paraffin-embedded sections of gastric tissues were fixed with cold methanol at 4℃ for 30 minutes, and then permeabilized with 0.2% Triton X-100 in phosphate-buffered saline (PBS) at room temperature for 10 minutes. The sections were stained with anti-periostin antibody (1:100), anti-α-SMA antibody (1:400) and the appropriate Alexa-Fluor-conjugated secondary antibodies (1:200), followed by counterstaining with a DNA-binding dye PI or DAPI (1 µg/mL in PBS) for 10 minutes. Fluorescence images were examined and photographed with the laser scanning confocal microscopy (Leica, Solms, Germany). About 8 vision fields were photographed randomly for each section. Each staining experiment was repeated at least 3–5 times.
Human gastric cancer cell lines MKN-45 and MGC-803 were cultured in an incubator at 37℃ in RPMI-1640 supplemented with 10% FBS with an atmosphere of 5% CO2. Prior to isoprenaline stimulation, cultures were incubated overnight in serum-free medium supplemented with 10 mM HEPES, 0.1% bovine serum albumin (pH 7.4). Cultures were then treated with 0, 5, or 10 uM isoprenaline for indicated time periods.
Sixty-five gastric cancer and 42 normal gastric tissue samples were collected from the General Hospital of PLA with informed consent of patients and the institutional approval. The real-time polymerase chain reaction (PCR) was performed using the Mx3005P system (Stratagene) with a 10 µL of TaqMan Gene Expression Master Mix, 20 µL of reaction volume containing 5 µL of cDNA, 1 µL of TaqMan Gene Expression Assay primers and probes for periostin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers for the genes of interest were: periostin193 bp (5'-GCCATCACATC GGACATA-3' and 5'-CTCCCATA ATAGACTCAGAACA-3'), and GAPDH 266 bp (5'-AGAA GGCTGGGGCTCATTTG-3' and 5'-AGGGGCCATCCACAGTCTTC-3'). The results were analyzed using the comparative threshold cycle (CT) method and normalized by a housekeeping gene GAPDH.
Total proteins from cells were extracted with a ice-cold lysis buffer. The concentration of proteins in the supernatant was analysed by the bicinchoninic acid method. Protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked with 5% slim milk in TBST at room temperature for 1 hour. After blocking, blots were probed overnight at 4℃ with a primary antibody diluted in blocking buffer (anti-periostin antibody 1:1000, anti-β-actin antibody 1:1000). The membranes were incubated with a horseradish peroxidase conjugated goat anti-rabbit/mouse secondary antibody (1:1000) for 2 hours at room temperature after washing three times with TBST. The membranes were developed using enhanced chemiluminescence substrate (Pierce) to transfer to film (Millipore).
Periostin has been characterized as a component of the tumor microenvironment, or metastatic niche, capable of promoting cancer development and progression. However, expression of periostin in gastric cancer has not been sufficiently studied. In this study, we first performed in situ hybridization in peri-cancerous, cancerous, and metastatic gastric cancer tissue samples to examine the expression patterns of periostin at the transcription level. In normal gastric tissues, periostin expression was not detected in gastric epithelial cells or the stroma of gastric tissues (Fig. 1A). In cancerous tissues, however, periostin was upregulated and present in the stroma of the primary tumors, but not in gastric epithelial cells (Fig. 1B). In metastatic gastric cancer tissues, periostin was also detected in lymphatic metastasis sections (Fig. 1C).
Stromal cells in gastric cancer produce periostin. Cancer-associated fibroblasts (CAFs), which interact with cancer cells to promote cancer progression, are identifiable by the expression of the myofibroblast marker α-SMA. Whether periostin in the cancer stroma is secreted by cancer cells or CAFs has not yet been determined in gastric cancer. We, therefore, collected gastric cancer tissue samples and used periostin combined with the fibroblast-associated protein α-SMA to label cancerous cells that co-expressed periostin and the fibroblast marker to evaluate the location of periostin in gastric cancer tissues. We first found that periostin expression correlated to the expression of α-SMA in gastric cancer tissues. Periostin was located mainly in the pericryptal fibroblasts (Fig. 2). In cancerous tissue where periostin was expressed at low levels, the expression of α-SMA was strongly correlated with the distribution patterns of periostin (Fig. 2A). In cancerous tissues where periostin showed high expression, α-SMA was also expressed at high levels in the membrane of cancerous gastric epithelial cells (Fig. 2B).
CAFs are the primary source of periostin, which facilitates tumor cell invasion by establishing a neoplastic niche in gastric cancers. Thus, we hypothesized that the niche increased its scope while the clinical staging of the tumor progressed. Given the active roles of periostin in regulating gastric cancer progression and metastasis, we then examined whether the distribution pattern of periostin was related to the clinical staging of tumors. Our results indicated that distribution patterns of periostin show a much broader distribution while the clinical staging of the tumor progressed (Fig. 3). Periostin is distributed at low levels in early gastric cancer (Fig. 3A). In intermediate gastric carcinoma, periostin shows a much broader distribution than in early gastric cancer (Fig. 3B). The distribution of periostin was the highest in late gastric cancer (Fig. 3C). These data suggest that the study of periostin may be a promising future research on the inhibition of gastric cancer progression and metastasis.
An emerging role for stress-related hormones in regulating cancer progression has been recognized. However, whether stress serves as a mechanism to promote gastric cancer development is not clear. To further investigate the relationship between periostin and stress-related hormones, we first performed real-time PCR to evaluate periostin expression after isoprenaline treatment. Thus, MKN-45 and MGC-803 cells were incubated with increasing doses of isoprenaline, and periostin was evaluated by real-time PCR. After incubation with 10 µM isoprenaline, periostin mRNA levels increased remarkably with a 4.9-fold enhancement in MKN-45 and 1.4-fold increase in MGC-803 gastric cancer cells compared with control groups (Fig. 4A and B). Next, MKN-45 and MGC-803 cells were stimulated with 0, 5, or 10 µM isoprenaline after serum starvation and periostin expression was evaluated by Western blot analysis (Fig. 4C and D). Isoprenaline stimulation of MGC-803 cells also upregulated periostin expression at both mRNA and protein levels in a time-dependent manner (Fig. 5). Collectively, these observations showed that isoprenaline is a positive regulator of periostin activity in gastric cancer cells.
Invasion and metastasis of tumor cells is the main cause of death in patients with gastric cancer.21 The metastatic niche plays important roles in cancer development and progression.22 Periostin, a major niche component, is functionally involved in multiple steps of cancer progression and participates in different signaling pathways, and thus is indispensable for gastric cancer progression and metastasis.23 In this study, we found that periostin is expressed in the stroma of the primary tumors and metastases, but not in normal gastric tissue. We also found that α-SMA expression was significantly correlated with periostin; periostin was located mostly in pericryptal fibroblasts, but not in tumor cells, and strongly correlated to the expression of α-SMA. Since periostin is believed to change quantitatively or qualitatively in normal, primary cancer, and metastatic niches, we then investigated whether the distribution patterns of periostin was related to the clinical staging of tumor by immunofluorescence, and found that the distribution pattern of periostin was broader as the clinical staging of the tumor progressed.
A growing list of molecular cues can initiate tumor development and progression.24 Recently, the emerging role of stress-related hormones in the regulation of cancer development and progression has been recognized.25 Epidemiological studies indicate that stress-related hormones might serve as risk factors for cancer development.26 Thus, we next examined the expression levels of periostin in gastric cancer cells at both mRNA and protein levels after treatment with different doses of stress-associated hormone isoprenaline, and surprisingly found that isoprenaline enhanced the expression of periostin in gastric cancer cell lines. Isoprenaline stimulation also upregulated periostin expression in a time-dependent manner in MGC-803 cells, indicating a direct connection between stress-related hormones and gastric cancer development and progression.
Our data together our data suggest that isoprenaline recognized and activated its target receptors, subsequently activating the downstream target periostin. The activation of periostin by isoprenaline functions as a regulator of subsequent cancer progression, and is associated with poor prognosis in human gastric carcinoma.23 Our findings provide a biochemical mechanism involved in psychosocial influences on gastric cancer pathogenesis, and suggest that pharmacological interventions targeting periostin or isoprenaline could potentially be used to ameliorate stress-associated influences on gastric cancer development and progression. As gastric cancer treatment develops towards a more patient-specific direction, consideration of the influence of psychosocial factors provides a novel perspective for new therapeutic targets.
Figures and Tables
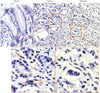 | Fig. 1Representative fluorescence images showing different expression patterns of periostin in peri-cancerous (A), cancerous (B), and metastatic gastric cancer tissue samples (C). In normal gastric tissues, periostin expression was absent in gastric epithelial cells and the stroma of gastric tissues (A). In cancerous tissues, periostin was upregulated and present in the stroma of primary tumors, but not in gastric epithelial cells (B). In metastatic gastric cancer tissues, periostin was detected in the areas of lymphatic metastasis (C). |
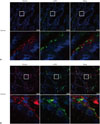 | Fig. 2Periostin expression correlated to the expression of fibroblast-associated protein α-smooth muscle actin (SMA) in gastric cancer tissues. Periostin was located in the pericryptal fibroblasts. (A and B) Representative fluorescence images show different expression patterns of periostin in gastric cancer tissue samples. In cancerous tissue where periostin was expressed at low levels, the expression of α-SMA strongly correlated with the distribution patterns of periostin (A). In cancerous tissues where periostin showed high expression, α-SMA was also expressed at high levels in the membrane of cancerous gastric epithelial cells (B). |
 | Fig. 3The distribution patterns of periostin show a much broader distribution while the clinical staging of the tumor progressed. Periostin was distributed at low levels in early gastric cancer (A). Periostin in intermediate gastric carcinoma shows a much broader distribution than in early gastric cancer (B). The distribution of periostin was highest in late gastric cancer (C). |
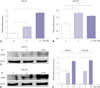 | Fig. 4Isoprenaline upregulated expression levels of periostin in gastric cancer cells. (A) Real-time PCR analyses of periostin expression in MKN-45 and MGC-803 cells after isoprenaline stimulation. Isoprenaline induced periostin expression in gastric cancer cells. (B) After incubation with 10 µM isoprenaline, periostin mRNA levels increased by 4.9-fold in MKN-45 and 1.4-fold in MGC-803 gastric cancer cells compared with control groups. (C and D) MKN-45 and MGC-803 cells were stimulated with 0, 5, or 10 µM isoprenaline after serum starvation. Periostin expression was analyzed by Western blot analysis. (E) Relative expression of periostin in gastric cancer cell lines after isoprenaline treatment. Data represent mean±SD (n=3 for each group, *p<0.05, †p<0.01, ‡p<0.001). POSTN, periostin. |
 | Fig. 5Isoprenaline upregulated periostin expression in MGC-803 gastric cancer cells in a time-dependent manner. (A) MGC-803 cells were starved overnight, and then treated with 5 µM isoprenaline for 0, 1, 2, 3, and 6 hour. The relative expression of periostin in MGC-803 cells was evaluated by real-time PCR. The results show that isoprenaline induced periostin expression in MGC-803 gastric cancer cells in a time-dependent manner. (B) Western blot analysis show that isoprenaline stimulation also upregulated periostin expression in a time-dependent manner in MGC-803 gastric cancer cells. (C) Relative expression of periostin in gastric cancer cells after isoprenaline treatment for indicated time periods. Data represent means±SD (n=3 for each group, *p<0.05, †p<0.01, †p<0.001). POSTN, periostin. |
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China, NO. 30901564, NO. 81101883, NO. 81372067, NO. 81121004, NO. 81230041; National Basic Science and Development Programme (973 Programme) NO. 2012CB518105; Beijing Novel Program, NO. 2008B53, NO. 2009A38.
References
1. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014; 20:1635–1649.

2. Neri S, Hashimoto H, Kii H, Watanabe H, Masutomi K, Kuwata T, et al. Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. J Cancer Res Clin Oncol. 2016; 142:437–446.

3. Scherzer MT, Waigel S, Donninger H, Arumugam V, Zacharias W, Clark G, et al. Fibroblast-derived extracellular matrices: an alternative cell culture system that increases metastatic cellular properties. PLoS One. 2015; 10:e0138065.

4. Bhakta G, Lim ZX, Rai B, Lin T, Hui JH, Prestwich GD, et al. The influence of collagen and hyaluronan matrices on the delivery and bioactivity of bone morphogenetic protein-2 and ectopic bone formation. Acta Biomater. 2013; 9:9098–9106.

5. Nissinen LM, Kähäri VM. Collagen turnover in wound repair--a macrophage connection. J Invest Dermatol. 2015; 135:2350–2352.

6. Williams PA, Silva EA. The role of synthetic extracellular matrices in endothelial progenitor cell homing for treatment of vascular disease. Ann Biomed Eng. 2015; 43:2301–2313.

7. Miles FL, Sikes RA. Insidious changes in stromal matrix fuel cancer progression. Mol Cancer Res. 2014; 12:297–312.

8. Liu Y, Liu BA. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol. 2011; 17:2674–2680.

9. Li JS, Sun GW, Wei XY, Tang WH. Expression of periostin and its clinicopathological relevance in gastric cancer. World J Gastroenterol. 2007; 13:5261–5266.

10. Contié S, Voorzanger-Rousselot N, Litvin J, Clézardin P, Garnero P. Increased expression and serum levels of the stromal cell-secreted protein periostin in breast cancer bone metastases. Int J Cancer. 2011; 128:352–360.

11. Liu Y, Shi J, Chen M, Cao YF, Liu YW, Pan J, et al. Periostin: a novel prognostic predictor for meningiomas. J Neurooncol. 2015; 121:505–512.

12. Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol. 2014; 184:859–870.

14. Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, et al. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem. 2015; 36:799–809.

15. Ratajczak-Wielgomas K, Dziegiel P. The role of periostin in neoplastic processes. Folia Histochem Cytobiol. 2015; 53:120–132.

16. Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011; 481:85–89.

17. Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011; 459:465–475.

18. Kashima TG, Nishiyama T, Shimazu K, Shimazaki M, Kii I, Grigoriadis AE, et al. Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol. 2009; 40:226–237.

19. Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006; 66:6928–6935.

20. Zhu S, Belkhiri A, El-Rifai W. DARPP-32 increases interactions between epidermal growth factor receptor and ERBB3 to promote tumor resistance to gefitinib. Gastroenterology. 2011; 141:1738–1748.e1-2.

21. Neverauskiene S, Machtejeviene E, Vaitkiene D, Juodzbaliene EB. [Disseminated ovarian, bone, and bone marrow metastases from gastric cancer]. Medicina (Kaunas). 2006; 42:923–931.
22. Pein M, Oskarsson T. Microenvironment in metastasis: roadblocks and supportive niches. Am J Physiol Cell Physiol. 2015; 309:C627–C638.

23. Liu GX, Xi HQ, Sun XY, Wei B. Role of periostin and its antagonist PNDA-3 in gastric cancer metastasis. World J Gastroenterol. 2015; 21:2605–2613.

24. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011; 147:275–292.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download