1. Esmaeli B, Ahmadi MA, Youssef A, Diba R, Amato M, Myers JN, et al. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg. 2004; 20:22–26.

2. Florentine BD, Fink T, Avidan S, Braslavsky D, Raza A, Cobb CJ. Extra-salivary gland presentations of adenoid cystic carcinoma: a report of three cases. Diagn Cytopathol. 2006; 34:491–494.

3. Matsuba HM, Simpson JR, Mauney M, Thawley SE. Adenoid cystic salivary gland carcinoma: a clinicopathologic correlation. Head Neck Surg. 1986; 8:200–204.

4. Khan AJ, DiGiovanna MP, Ross DA, Sasaki CT, Carter D, Son YH, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001; 96:149–158.
5. Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992; 164:623–628.

6. Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D'Amico F. Risk factors for local recurrence of adenoid cystic carcinoma: the role of postoperative radiation therapy. Am J Otolaryngol. 1999; 20:281–286.

7. Li N, Xu L, Zhao H, El-Naggar AK, Sturgis EM. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012; 118:3945–3953.

8. Polito E, Leccisotti A. Epithelial malignancies of the lacrimal gland: survival rates after extensive and conservative therapy. Ann Ophthalmol. 1993; 25:422–426.
9. Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995; 32:619–626.

10. Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979; 39:1141–1149.
11. Su F, Hu X, Jia W, Gong C, Song E, Hamar P. Glutathion S transferase pi indicates chemotherapy resistance in breast cancer. J Surg Res. 2003; 113:102–108.

12. Huang J, Tan PH, Thiyagarajan J, Bay BH. Prognostic significance of glutathione S-transferase-pi in invasive breast cancer. Mod Pathol. 2003; 16:558–565.

13. Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013; 9:1292–1307.

14. Gibson SB. A matter of balance between life and death: targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy. Autophagy. 2010; 6:835–837.

15. Dewaele M, Maes H, Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010; 6:838–854.

16. Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009; 11:777–790.

17. Bellot GL, Liu D, Pervaiz S. ROS, autophagy, mitochondria and cancer: Ras, the hidden master? Mitochondrion. 2013; 13:155–162.

18. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008; 132:27–42.

19. Li L, Ishdorj G, Gibson SB. Reactive oxygen species regulation of autophagy in cancer: implications for cancer treatment. Free Radic Biol Med. 2012; 53:1399–1410.

20. Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007; 7:961–967.

21. Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011; 8:528–539.

22. Roy S, Debnath J. Autophagy and tumorigenesis. Semin Immunopathol. 2010; 32:383–396.

23. Jiang L, Huang S, Zhang D, Zhang B, Li K, Li W, et al. Inhibition of autophagy augments chemotherapy in human salivary adenoid cystic carcinoma. J Oral Pathol Med. 2014; 43:265–272.

24. Jiang LC, Huang SY, Zhang DS, Zhang SH, Li WG, Zheng PH, et al. Expression of beclin 1 in primary salivary adenoid cystic carcinoma and its relation to Bcl-2 and p53 and prognosis. Braz J Med Biol Res. 2014; 47:252–258.

25. Wang YF, Zhang W, He KF, Liu B, Zhang L, Zhang WF, et al. Induction of autophagy-dependent cell death by the survivin suppressant YM155 in salivary adenoid cystic carcinoma. Apoptosis. 2014; 19:748–758.

26. Chen G, Hu X, Zhang W, Xu N, Wang FQ, Jia J, et al. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis. 2012; 17:90–101.

27. Szanto PA, Luna MA, Tortoledo ME, White RA. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984; 54:1062–1069.

28. Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010; 41:107–112.

29. Brockstedt U, Krajinovic M, Richer C, Mathonnet G, Sinnett D, Pfau W, et al. Analyses of bulky DNA adduct levels in human breast tissue and genetic polymorphisms of cytochromes P450 (CYPs), myeloperoxidase (MPO), quinone oxidoreductase (NQO1), and glutathione S-transferases (GSTs). Mutat Res. 2002; 516:41–47.

30. Van Emburgh BO, Hu JJ, Levine EA, Mosley LJ, Case LD, Lin HY, et al. Polymorphisms in drug metabolism genes, smoking, and p53 mutations in breast cancer. Mol Carcinog. 2008; 47:88–99.

31. Chekhun VF, Zhylchuk VE, Lukyanova NY, Vorontsova AL, Kudryavets YI. Expression of drug resistance proteins in triple-receptor-negative tumors as the basis of individualized therapy of the breast cancer patients. Exp Oncol. 2009; 31:123–124.
32. Chaiwun B, Sukhamwang N, Trakultivakorn H, Saha B, Young L, Tsao-Wei D, et al. GSTPi-positive tumour microenvironment-associated fibroblasts are significantly associated with GSTPi-negative cancer cells in paired cases of primary invasive breast cancer and axillary lymph node metastases. Br J Cancer. 2011; 105:1224–1229.

33. Dhar SK, St Clair DK. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. 2012; 52:2209–2222.

34. Oberley LW, Bize IB, Sahu SK, Leuthauser SW, Gruber HE. Superoxide dismutase activity of normal murine liver, regenerating liver, and H6 hepatoma. J Natl Cancer Inst. 1978; 61:375–379.
35. Coursin DB, Cihla HP, Sempf J, Oberley TD, Oberley LW. An immunohistochemical analysis of antioxidant and glutathione S-transferase enzyme levels in normal and neoplastic human lung. Histol Histopathol. 1996; 11:851–860.
36. Van Driel BE, Lyon H, Hoogenraad DC, Anten S, Hansen U, Van Noorden CJ. Expression of CuZn- and Mn-superoxide dismutase in human colorectal neoplasms. Free Radic Biol Med. 1997; 23:435–444.

37. Czeczot H, Skrzycki M, Podsiad M, Gawryszewska E, Nyckowski P, Porembska Z. [Antioxidant status of patients with primary colorectal cancer and liver metastases of colorectal cancer]. Pol Merkur Lekarski. 2005; 18:58–61.
38. Sun GG, Wang YD, Chen LQ, Wang SJ, Liu GL, Yu XR, et al. Novel cancer suppressor gene for esophageal cancer: manganese superoxide dismutase. Dis Esophagus. 2011; 24:346–353.

39. Kocot J, Kiełczykowska M, Da¸browski W, Piłat J, Rudzki S, Musik I. Total antioxidant status value and superoxide dismutase activity in human colorectal cancer tissue depending on the stage of the disease: a pilot study. Adv Clin Exp Med. 2013; 22:431–437.
40. Tamura M, Matsui H, Tomita T, Sadakata H, Indo HP, Majima HJ, et al. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J Clin Biochem Nutr. 2014; 54:12–17.

41. Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer. 2005; 116:340–350.

42. Liang LZ, Ma B, Liang YJ, Liu HC, Zheng GS, Zhang TH, et al. High expression of the autophagy gene Beclin-1 is associated with favorable prognosis for salivary gland adenoid cystic carcinoma. J Oral Pathol Med. 2012; 41:621–629.

43. Zhao H, Yang M, Zhao J, Wang J, Zhang Y, Zhang Q. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol. 2013; 30:475.

44. Karpathiou G, Sivridis E, Koukourakis M, Mikroulis D, Bouros D, Froudarakis M, et al. Autophagy and Bcl-2/BNIP3 death regulatory pathway in non-small cell lung carcinomas. APMIS. 2013; 121:592–604.

45. Jin T, Lin HX, Lin H, Guo LB, Ge N, Cai XY, et al. Expression TGM2 and BNIP3 have prognostic significance in laryngeal cancer patients receiving surgery and postoperative radiotherapy: a retrospective study. J Transl Med. 2012; 10:64.

46. Koop EA, van Laar T, van Wichen DF, de Weger RA, Wall Ev, van Diest PJ. Expression of BNIP3 in invasive breast cancer: correlations with the hypoxic response and clinicopathological features. BMC Cancer. 2009; 9:175.

47. Liao W, Sun L, Wang C, Huang H, Liu J, Liao W, et al. LC3A-positive "stone-like" structures predict an adverse prognosis of gastric cancer. Anat Rec (Hoboken). 2014; 297:653–662.

48. Spowart JE, Townsend KN, Huwait H, Eshragh S, West NR, Ries JN, et al. The autophagy protein LC3A correlates with hypoxia and is a prognostic marker of patient survival in clear cell ovarian cancer. J Pathol. 2012; 228:437–447.

49. Karpathiou G, Sivridis E, Koukourakis MI, Mikroulis D, Bouros D, Froudarakis ME, et al. Light-chain 3A autophagic activity and prognostic significance in non-small cell lung carcinomas. Chest. 2011; 140:127–134.

50. Levy JM, Thorburn A. Targeting autophagy during cancer therapy to improve clinical outcomes. Pharmacol Ther. 2011; 131:130–141.

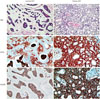


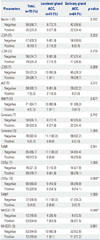
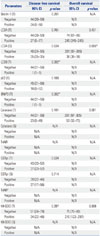





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download