INTRODUCTION
Hepatocellular carcinoma (HCC) constitutes one of the most common gastrointestinal malignancies worldwide, and its incidence continues to increase.
1 The historical role of radiation therapy (RT) in HCC patients has been insignificant due to the low radiation tolerance of the whole liver. However, radiotherapeutic technical advances using three-dimensional conformal RT (3D-CRT), intensity-modulated RT (IMRT) and image-guided RT (IGRT) technology have considerably contributed to the improvement of therapeutic ratios in terms of the administration of higher radiation doses to the target volumes and significant reduction in surrounding normal tissue complications.
123
In locally-advanced-stage HCC, the frequency of uncontrolled primary tumors after previous irradiation or sustained symptomatic local problems remains high. Thus, RT currently plays an important role in locally-advanced as well as early-stage, locally-confined liver tumors. The need for re-irradiation as a salvage option in HCC patients is also continuously increasing.
Despite the development of modern RT technology, re-irradiation in HCC remains a challenging issue due to the relatively low radiation tolerance of the small and large bowels and the continuous target motion around the diaphragm or normal bowels.
45 Another remaining problem for re-irradiation is the strong necessity of an accurate tool for determining radiation dose summation. Fortunately, commercially available software that applies an intensity-based free-form deformable registration algorithm has been utilized at several institutions recently.
678 Therefore, conceptually, we can perform re-irradiation more safely than before by using a more conformal treatment planning method involving a novel RT technique and by more accurately estimating cumulative radiation doses using a deformable image registration (DIR) tool.
In the present study, we sought to evaluate the potential clinical applicability of the DIR method in HCC re-irradiation by analyzing the dosimetric results, clinical outcomes, and toxicities of re-irradiation cases. Based on the study results, we suggest future perspectives on re-irradiation for HCC using DIR.
MATERIALS AND METHODS
Patient population
Between August 2010 and March 2012, 12 eligible patients received re-irradiation for HCC using helical tomotherapy (HT)-based IMRT and were included in this study. We obtained the approval of the Institutional Review Board of Severance Hospital to conduct this study. The eligibility criteria of the study were as follows: re-irradiation using HT in HCC; re-irradiation delivery of full tolerable RT doses with normal tissue constraints; re-irradiation interval of more than 5 months from the first course of RT; and adequate hepatic function and general performance status allowing toleration of the entire RT course. Patients who received additional small doses of RT with 3D-CRT or HT, which was regarded as boost RT for persistent diseases, within 1-3 months after the first course of irradiation were excluded from this analysis.
Radiotherapy
Simulation of 3D-CRT was routinely performed in the same manner, in the supine position. To assess the respiration-associated margins, fluoroscopic examination was regularly conducted before 2011. Four-dimensional computed tomography (4D-CT) was introduced after 2011 and has been commonly used to evaluate the movement of the targets using a SOMATOM (Siemens, Berlin, Germany) CT scanner.
Prior to 2012, simulation of HT was performed using only the BodyFix system (Medical Intelligence, GmbH, Schwabmunchen, Germany) to immobilize the patients, and the upper-abdominal area was compressed by elastic foils using low negative pressure in order to restrict body motion. Beyond that period, abdominal compressors, which directly compress the upper-abdominal areas below the xiphoid process, were utilized with the BodyFix system to minimize the respiration-associated margins.
During HT, the simultaneous integrated boost (SIB) technique, which prescribes different fractional doses in gross tumor volume (GTV) and clinical target volume (CTV), was commonly utilized. During re-irradiation, we were commonly confronted with problems of overdose in organs-at-risk (OARs); this was countered by routine adaptive planning, which reduced the treatment of target volumes after tumor shrinkage or reduced planning target volume (PTV) margins in close proximity to the critical organs.
Prescribed dose summation constraints for OARs were as follows: ≤50 Gy per 2 cc of small and large bowels; ≤45 Gy per 2 cc of duodenum and stomach; and mean remaining liver dose (MRLD) ≤30 Gy, with at least 700 cc of remaining liver volume. However, when we were unable to comply with those dose constraints, we endeavored to prescribe the lowest doses possible to the OARs.
Target volume and critical organ assessment
OARs were slightly different according to the location of tumors. For example, re-irradiation was relatively safe in tumors located around the liver dome or right lobe, and the liver itself was the main dose-limiting organ. In contrast, the neighboring bowels, duodenum, or stomach were the dose-limiting OARs in tumors located in the left lobe or lower portion of the liver. The purpose of this study was to re-evaluate the dosimetric summation results in critical organs; thus, we assessed the dosimetric factors of the most important OARs for re-irradiation including the liver, bowels, duodenum, and stomach.
The dosimetric data were retrospectively retrieved from actual registered treatment records. At the time of re-irradiation, we could only approximately estimate the cumulative dose of critical organ using the Pinnacle (Philips, Madison, WI, USA) RT planning system. Each contoured organ was recalled to the software, and the OARs were newly contoured if needed. The VRL was defined as the total liver volume (VTL) minus CTV. CTV was defined as the treated GTV plus a margin of 0.3-0.5 cm. Bowels were defined as the total bowels including the small and large bowels, which were shown in the planning CT.
Deformable image registration method
DIR was performed using the software MIMvista version 5.2 (MIM Software, Inc., Cleveland, OH, USA), which utilizes an intensity-based, free-form deformable registration algorithm. We chose the chronological dose summation scenario for this study, as this method reflected the latest information about each anatomical structure and was considered to be more reliable than the anti-chronological version. In the registration process, we the adjustment of important OARs was given priority. Deformed prescribed doses were summed with the doses of the re-irradiation plan in the same manner as previously described.
8 Dose summation was performed by both rigid (uniform transformation of all voxels) and deformable (deformable transformation based on voxel similarity) registration methods. RT dose levels were evaluated on dose-volume histograms. To evaluate the detailed dosimetric analyses, the cumulative D
max (ΣD
max), D
0.1 cc (ΣD
0.1 cc), D
1 cc (ΣD
1 cc), and D
2 cc (ΣD
2 cc) in each OAR were calculated. Dx cc was defined as the RT dose receiving X cc.
Evaluation of clinical outcome and toxicity
Follow-up was regularly conducted after 1 month initially and then at 3- to 6-month intervals. Complete blood counts, liver function tests, and other important blood chemistry tests were performed every 1 to 2 weeks or more frequently during treatment, and at 1-, 3-, and 6-month intervals thereafter.
To evaluate the treatment response, modified RECIST (mRECIST) criteria and RECIST criteria were utilized for HCC parenchymal lesions and abdominal lymph nodes (LNs), respectively.
9 Evaluation of major treatment responses was conducted for in-field lesions. The timing of the response achievement was recorded as the earliest period of maximal tumor response, which was evaluated by the aforementioned criteria. Change in liver function was assessed based on the Child-Pugh (C-P) classification. We hypothesized that major liver function change after RT could occur within early periods, and we evaluated the change in the C-P score for up to 6 months after each course of RT. In this study, we aimed to determine the safety of re-irradiation, and severe toxicity (≥grade 3) was evaluated. The criteria of toxicity were evaluated using the Common Toxicity Criteria for Adverse Events (CTCAE) version 4.
Statistical analyses
All statistical analyses were conducted using PASW statistics 18 (SPSS Inc., Chicago, IL, USA). A non-parametric Wilcoxon rank-sum test was utilized to discriminate the difference between the two different types of dose summation methods (rigid vs. deformable). Fisher's exact test was used to compare the distribution of variables. A p-value <0.05 was considered to be statistically significant, and all statistical analyses were based on the two-sided test.
RESULTS
Patient and treatment characteristics
The patient characteristics at the first course of RT and at re-irradiation are summarized in
Table 1 and
2, respectively. The first course of RT was delivered by 3D-CRT (n=9) or HT (n=3). Concurrent chemotherapy (5-FU) was employed with RT in five patients, and nine patients received transarterial chemoembolization (TACE), doxorubicin-eluting bead TACE (DC-bead TACE) or transarterial chemoinfusion (TACI) as combined treatment modalities. Before the first course of RT, the majority of the patients (11 of 12) had received other localized or systemic treatments. The median primary prescribed total RT dose and daily fractional dose were 50 Gy (range, 36-60 Gy) and 1.8 Gy (range, 1.8-3 Gy), respectively. Re-irradiation was performed with a median total dose of 50 Gy (range, 36-58.42 Gy) and a median fractional dose of 2.54 Gy (range, 2.5-9 Gy). The median elapsed time from the first course of RT to re-irradiation was 20.3 months (range, 5.3-69.4 months). Most of the re-irradiation fields were previously irradiated or were marginal areas in relation to the initial course of RT.
After re-irradiation, three patients (patients 1, 4, and 5) received sorafenib for suspicious remnant lesions, and one patient (patient 2) received 5-FU-based systemic chemotherapy after extrahepatic disease progression. One patient (patient 10) received ten cycles of TACI after re-irradiation.
Therapeutic effect of RT
Treatment responses after the first irradiation and re-irradiation are described in
Table 1 and
4, respectively. In-field tumor response [complete response (CR)/partial response (PR)/stable disease (SD)] rates after the first and second courses of RT were 100% and 90.9%, respectively. One patient (patient 4) showed disease progression 3 months after re-irradiation, and we were unable to evaluate a response from one patient (patient 3) due to death before response assessment.
Dose summation indices for the liver
Table 3 shows the results of liver volume and liver dose summation indices.
The median VTL and the median VRL at the initial course of RT were 1531.1 cc (range, 1107.8-3041.3 cc) and 1131 cc (range, 986.3-1560.9 cc), respectively. The median VTL and the VRL at the time of re-irradiation were 1157.8 cc (range, 887.9-2078.9 cc) and 964.5 cc (range, 746.6-1335.7 cc), respectively. The median ΣMRLD for rigid- and deformable-type registration were 28.3 Gy (range, 17.4-48.2 Gy) and 27.7 Gy (range, 17.2-42.7 Gy), respectively, and the results for the two different DIR methods did now show statistical difference (p=0.248, Wilcoxon ranksum test). In patients who showed a large change in VTL (Δ-VTL) between time intervals (patients 4, 5, 8, and 11), the MRLD (Δ-MRLD) indices of the two different DIR methods also showed a large difference.
In patients 4 and 11, the calculated ΣMRLD was overestimated compared to each course of MRLD due to a large CTV at the initial course and large differences in CTV between each course of RT. In this situation, the calculated ΣMRLD could be overestimated compared to its predicted value, and a separate assessment of ΣMRLD in each course was mandatory. In patient 10, VTL and VRL in the initial course did not show a large difference as the main target was the portocaval LN.
Dose summation indices for other OARs
We assessed the usefulness of DIR methods by dosimetric calculation and visual review. We visually inspected whether the D
max points were actually in the predicted areas for each OAR. The actual D
max dose summation results (initial D
max+re-irradiation D
max) from each course of RT were compared to the summated dose (ΣD
max) calculated by MIMvista. Among 36 available indices for OARs (bowel, duodenum, and stomach doses in each patient) dose summation (
Table 4), 12 indices (33.3%) were useful for utilizing DIR, and 21 (58.3%) were useless. The remaining three indices (8.3%) showed contradictory results (different results for rigid and deformable types). There was no statistical difference between the two different DIR methods (rigid and deformable types) in terms of ΣD
0.1 cc, ΣD
1 cc, ΣD
2 cc, and ΣD
max for each OAR.
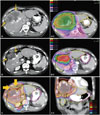
A representative illustrative case (patient 4) is shown in
Fig. 1.
Toxicity and evaluation of a causal relationship
Liver function change as determined by C-P classification was observed in four and eight patients at the first and second courses of RT, respectively. Deterioration of liver function was found in two patients at the first course of RT (patients 4 and 5) (
Table 1); however, this change was only a minor worsening of the C-P score. We also observed two patients who showed mild C-P score recovery. After re-irradiation, the majority of patients (6 of 12) showed deterioration of liver function, and severe worsening of liver function (C-P score elevation ≥3 points) was also observed in four patients (patients 3, 4, 6, and 9) (
Table 5). In patients 3, 4, and 6, the causes of deaths were lung, liver abscess, and pleural effusion (patient 3), hepatorenal syndrome and hepatic failure (patient 4), and liver function deterioration and related superimposed septic shock (patient 6). Among four patients who presented with a C-P score elevation ≥3 points, only one patient (patient 4) showed a high deformable ΣM
RLD index of 42.7 Gy. A C-P score elevation ≥2 points tended to be significantly associated with the use of TACE or DC-bead TACE as a combined treatment modality at re-irradiation (
p=0.061, Fisher's exact test). The median value of deformable ΣM
RLD was not significantly associated with severe C-P score elevation (ΣM
RLD in patients with C-P score elevation: ≥2 vs. <2,
p=0.415; ΣM
RLD in patients with C-P score elevation: ≥3 vs. <3,
p=0.465).
Patient 1 was diagnosed with gastric and duodenal ulcers 4 months before re-irradiation and was treated for Helicobacter pylori eradication. However, duodenal ulcer perforation suddenly developed 20 months after re-irradiation and was managed by emergency operation. The patient's duodenal ΣDmax and ΣD0.1 cc by deformable type DIR were 70.7 Gy and 68.4 Gy, respectively.
DISCUSSION
The present study evaluated the feasibility of using a DIR method in the re-irradiation of HCC. Several publications reported the clinical utility of DIR algorithms in the head and neck regions.
678 This method uses a deformation map of vector fields by connecting each voxel from the initial CT to the second course of CT. By assigning manually-contoured structures and planned RT doses to the corresponding voxels, DIR can be used to correct the time- and spatially-dependent distribution differences.
10 Clinical application of this algorithm has been evaluated, and the acceptable error rates in consistency and reproducibility have been demonstrated in head and neck areas.
7 However, in the study by Brock, et al.,
11 the accuracy results from one anatomical site did not translate to another site, particularly in the abdominal organs. A three-dimensional deformable phantom study performed by Juang, et al.
12 also showed significant errors in dose mapping using commercially available deformable registration algorithms.
Dosimetric study in the abdomen using this tool has not yet been reported. This is probably due to the unique features of uncertainty in abdominal areas, characterized by continuous movement of target volumes caused by normal bowel motion and respiration. Therefore, we could not convincingly demonstrate that our dose summation method using DIR was rational or could be used practically in these highly variable regions. However, we hypothesized that OARs-centered DIR may perform better than not using any dose summation engines in the re-irradiation setting in terms of dose summation calculation. In addition, we were frequently confronted with challenging re-irradiation cases of HCC in the clinic and needed to examine the dosimetric analyses using this tool, which was the main purpose of this pilot study. All recruited patients had been reirradiated using HT, and commercially available DIR software was utilized to represent dose summation indices.
Re-irradiation in HCC was mainly administered as the last salvage option when the tumor was localized and there were no other possible treatment modalities. Despite re-irradiation dose limits to the in-field area, full tolerable doses were employed, and the overall response rate of 90.9% was encouraging; moreover, PR could be achieved in three patients (27.3%) in our study. However, one duodenal perforation and four cases of severe C-P score elevation developed.
When planning the treatment, we attempted to preserve the MRLD based on the critical volume model of liver.
13 Liver function significantly deteriorated after re-irradiation at a relatively high frequency, whereas an evident causal dose-response was not identified. The consecutive employment of other combined treatments (TACE and DC-bead TACE) at re-irradiation was the main contributing factor for the development of severe liver function damage. This was probably due to the extensive liver damage caused by TACE, which could be aggravated by vascular injuries that were already formed at the first course of irradiation. One case of duodenal ulcer perforation was identified; however, that patient already had a history of duodenal and gastric ulcers before re-irradiation, and ΣD
max and ΣD
0.1 cc indices by deformable-type DIR were much higher than the tolerable doses. Bae, et al.
14 reported the clinical importance of ulcer history in the development of severe duodenal toxicity following gastrointestinal stereotactic body radiotherapy, and the results were concordant with our experience.
In terms of intestinal OAR doses, we endeavored to adhere to normal tissue constraints in re-irradiation using stepwise adaptive planning. Although there were several patients who received significant cumulative doses in limited volumes of OARs, we did not observe clinically significant toxicities in all but one of these patients. However, the follow-up duration was limited, and additional follow-up in good performance future cohorts is needed to confirm the results.
In modern DIR calculations, different registration methods (rigid vs. deformable) did not show statistically different results in terms of dose summation for either solid (liver) or hollow (bowels, duodenum, stomach) OARs. However, when Δ-VTL was large between intervals, the Δ-MRLD between the two different types of DIR also seemed to increase. In hollow OARs, the dose summation using DIR revealed somewhat variable results, with 58.3% being useless. Moreover, visual inspections in each region were essential to confirming the summation results. Thus, the modern DIR method might be more reliably utilized in solid organs, which have a limited spatial difference between intervals in terms of summated dose calculation, after careful visual review.
We aimed to investigate the clinical correlation between normal tissue dose summation results and high-degree clinicofunctional deteriorations. Although we did not find a dosimetric causal relationship, subclinical mucosal changes in OARs could be substantially high, as previously reported.
15 Moreover, in cirrhotic HCC patients, the frequency of undetectable mucosal changes was commonly noticed, and special concern is required in this population.
1516 More intensive review involving endoscopic exams will disclose the subclinical toxicities in future studies.
In conclusion, the OARs-based, DIR-based dose summation method can be utilized in re-irradiation of HCC patients after careful visual confirmation of high-risk regions. Although we did not reach optimal and accurate dose summation results under the current algorithms and circumferential uncertainties, we can conclude that more favorable gastrointestinal toxicity profiles are expected with this modern technology. DIR may be more reliably used in solid organs with limited spatial difference between intervals in comparison to the hollow organs, in terms of accurate prediction of dose summation. A more optimized adaptive plan using a highly conformal RT technique and appropriate use of DIR may enhance radiotherapeutic outcomes in future HCC cohorts treated with re-irradiation.









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download