Abstract
Purpose
The objective was to determine the characteristics and prognostic factors of 86 Chinese patients with trisomy 8 aberrations and compare the prognostic value of International Prognostic System (IPSS) and Revised IPSS (IPSS-R) in this cohort.
Materials and Methods
A total of 86 cases diagnosed with primary myelodysplastic syndromes (MDS) with isolated tr8 or with tr8 and other additional cytogenetic aberrations diagnosed and treated at the Union Hospital, Tongji Medical College of Huazhong University of Science and Technology between July 2002 and March 2013 were reviewed.
Results
The median survival of the entire group was 23.0 months, and acute myeloid leukemia (AML) developed in 43% (37/86) patients within the follow up time. The univariate analysis revealed that overall survival (OS) was correlated with age, thrombocytopenia, absolute neutrophil count, marrow blasts, cytogenetic status and red blood cell transfusion at diagnosis, and the multivariate analysis revealed that age, marrow blasts, cytogenetic status and transfusion dependence were independent parameters for the OS. The cytogenetic complexity and marrow blasts had the strongest impact on the AML transformation by multivariate analysis. Comparing the two prognostic systems, both two systems could successfully discriminate risk groups for survival. IPSS-R was more refined than IPSS for predicting OS, but had no advantage in predicting the risk of AML development.
Myelodysplastic syndrome (MDS) is a group of malignant clonal diseases originating from hematopoietic stem cells and characterized by pathophysiological changes in dysplasia and ineffective hematopoiesis of clonal hematopoietic stem cells. Its clinical manifestations include cytopenia of one or more lineages, dysplasia of cells in bone marrow and peripheral blood, and an obvious tendency to transform into acute leukemia, particularly in more advanced forms of MDS.1234 The overall survival (OS) and risk of leukemic transformation in such patients are highly variable.56789 The International Prognostic Scoring System (IPSS) has widely been used as the golden prognostic classification with primary MDS.10
Recently, the newer prognostic system Revised IPSS (IPSS-R) was proposed.8 Both systems indicate that cytogenetics is an independent prognostic factor of MDS concerning OS and acute myeloid leukemia (AML) transformation.910
Trisomy 8 (tr8 or +8) is the most common chromosomal gain in MDS, which is present in 5% of all MDS patients in Western countries.11 However, it accounts for about 30–35% in Chinese patients with abnormal karyotype and represents 14–20% of MDS patients in Western countries.121314 Although tr8 as sole anomaly is categorized as intermediate IPSS cytogenetic subgroup, the outcome varies greatly both in terms of OS and risk of evolution to AML.51516 Furthermore, patients with trisomy 8 are more likely to improve hematologically with immunosuppressive treatment (IST) compared with patients with other forms of MDS.17 Recently, the optimal therapies for the trisomy 8 patients have been investigated.18 Thus, the analysis of further prognostic parameters for OS and AML transformation in large series of MDS patients with trisomy 8 is of importance. There are few studies that have systematically analyzed the clinical prognostic factors of primary MDS with tr8 in Chinese patients.121315 In this study, we aimed to assess clinical features and identify prognostic factors in Chinese primary MDS with tr8 anomaly, and compare the prognostic value of IPSS and IPSS-R in this group by our single institution cohort.
We retrospectively analyzed 86 (23%) patients with MDS from a series of 374 cases diagnosed and followed up in our institution between July 2002 and March 2013. A total of 86 cases diagnosed with MDS with isolated tr8 or with tr8 and other additional cytogenetic aberrations were enrolled for the study. The diagnosis of the patients were reviewed, and reclassified according to World Health Organization (WHO) classification of 2008,16 and the exact date of diagnosis with bone marrow examination and cytogenetic assessment had to be documented. Exact WHO type, medullary blast count, differential count, bl-ood cell counts, karyotype, and transfusion requirement were documented. Transfusion dependency means that at least 4 units of red blood cell (RBC) transfusions had to be administered within 8 weeks, which was defined as described by Malcovati, et al.19 As a result, 86 patients had met the criteria and were enrolled. The studies had been approved by ethics committees of participating institutions and conducted in accordance with the Declaration of Helsinki. All participants had given informed consent.
All the bone marrow chromosome studies were performed by following chromosome-banding procedures, and at least 20 metaphases were analyzed. Abnormal clones were described in accordance with the 2006 International System for Human Cytogenetic Nomenclature (ISCN),20 and aberrations were counted following the International Working Group on MDS Cytogenetics (IWGMC) consensus guidelines.21 Fluorescence in situ hybridization (FISH) analysis was performed on short-term cultured bone marrow. Sample preparations and hybridizations using commercially available probes were performed according to manufacturer's recommendations (Vysis, Downers Grove, IL, USA). Systematic screening for del(5q), del(7q)/-7, +8, del(20q), and -Y was performed on each case. A minimum of 500 interphase cells were analyzed. If the cells with abnormal signal were less than 5%, 1000 inter-phase cells were screened. Normal control values were previously established by using five normal samples of bone marrow donors and 15 bone marrow samples of iron deficiency anemia patients with normal karyotype. Using the method of x±258 standard deviations (99% confidence interval), the cut-off level for normal range values was established for each probe as follows: del (5q)<0.5%; del(7q)/-7<0.2%; +8<0.5%; del(20q)<1%; and -Y<5%.
According to WHO classification, all patients were administered with supportive care. Patients were followed up with repeated examinations, including bone marrow aspirate, cytogenetics and molecular genetics, every 6 months or whenever any change in their clinical condition occurred. Follow-up was completed by the end of March 2013.
For the analysis of survival and AML development, patients who were still alive were censored at the date of last observation. Survival time was counted from diagnosis. OS was defined as the time from diagnosis to death or date of last follow-up. The time to AML transformation was defined as time to bone marrow blast increase to 20%, according to the WHO classification.22 OS was analyzed with the Kaplan-Meier method and followed by the log-rank.23 All univariate tests were two tailed and p value ≤0.05 was considered as significant. Finally, Cox proportional hazards multivariate model was used to define the OS and to assess the relevant prognostic variables.24 For multivariate analysis, a p value ≤0.05 was considered significant. All analyses were performed using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA).
The 86 patients with trisomy 8 abnormality were analyzed as a whole. Fifty-four patients were male (62.8%) and 32 were female (37.2%), with median age of 48.5 years (range of 21–79). The median hemoglobin level, absolute neutrophil count (ANC) and platelet count were 76.5 g/L (range of 42–134), 1.5×109/L (range of 0.08–10.1), and 64.5×109/L (range of 7–297), respectively. The median proportion of bone marrow blasts was 3% (range of 0–18) (Table 1). According to the IPSS, therefore, 76.8% had hemoglobin level <100 g/L, 54.7% had ANC <1.8×109/L and 55.8% had a platelet count <100×109/L. At the time of diagnosis, 35% patients regularly needed RBC transfusion. In total, 49 (57.0%) patients had a single trisomy 8 abnormality, while 19 (22.1%) had one additional aberration and 18 (20.9%) had more than three aberrations (complex karyotype including tr8). According to the 2008 WHO classification, the patients were classified as 19 cases of RCUD, 1 case of RARS, 25 cases of RCMD, 15 cases of RAEB1, 20 cases of RAEB2, and 6 cases of MDS-U.
The median follow-up time was 22 months. The median OS for our study group was 23.0 months. Univariate analysis by log-rank test was done to screen the parameters potentially associated with the time of survival (Table 1). Age above 60 years old, marrow blasts, cytogenetic complexity, ANC, platelet count (<50×109/L) and transfusion dependency at diagnosis were associated with a worse prognosis. Then, we further assessed the WHO Classification-Based Scoring System (WPSS) for the OS, and found that the intermediate group vs. high and very high group has significant difference (Fig. 1). In the multivariate analysis, age, marrow blasts, cytogenetic status and the need of RBC transfusion at diagnosis were independent parameters for the time of survival (Table 2). For the cytogenetic status, we found a difference in the time of survival between the karyotype complexity groups: the median time for the groups (tr8, tr8+1, and tr8+≥2) was 32.3, 22.5, and 11.9 months, respectively, with the difference being statistically significant between tr8 and tr8+1 or tr8+≥2 (p<0.05) or between tr8+1 and tr8+≥2 (p<0.05) (Fig. 2).
It was found that both IPSS and IPSS-R could successfully predict the OS (p<0.05), however, the IPSS has the advantage for predicting the AML transformation risk (p<0.05) than the IPSS-R (p=0.154). In the IPSS groups, there was no significant difference in the OS between intermediate-2 risk group and high risk groups (p=0.535) (Fig. 3). When the prognosis risk scores were recalculated according to the IPSS-R; 37.5% (18 cases) intermediate-1 from the IPSS group was redistributed into the IPSS-R low-risk group, 18.8% (9 cases) was into high-risk group; 48% (13 cases) intermediate-2 from the IPSS was put into the IPSS-R high-risk group, 30% (8 cases) intermediate-2 was put into very high-risk group, and 81.8% (9 cases)
During the entire follow time, there were 37 (43%) of the 86 patients progressed to AML. Then, we explored the influence of parameters on the risk of AML progression by univariate analysis, and found that cytogenetic status and marrow blasts >5% were significantly (p<0.05) associated with the increased risk of the AML transformation. In the multivariate analysis, the cytogenetic complexity and the count of bone marrow blasts were identified to be the independent risk factors for AML evolution (Table 3).
MDS is characterized by highly clinical heterogeneous behavior, and numerous studies have suggested that the Asian MDS patients are different from those in Western countries.25262728 The median age in our cohort was younger (48.5 years old) than the reported cases in the Western countries (72 years old). The incidence of the MDS with the tr8 abnormality is different between the Western and the Asian. In China, trisomy 8 is the most frequent karyotypic abnormality.1314293031 Furthermore, the clinical characteristic and prognostic factors of Asian MDS patients are different from Western patients.2632 Therefore, we considered that the prognostic parameters of this group may be different between the Westerners and Chinese.
As for the significance of clinical parameters for the OS and AML development in the present study, age, low platelet, and degree of ANC had an impact on OS but not on AML transformation. The significantly bad prognosis of low platelet count had been described in MDS patients.31 However, hemoglobin level had no significance (p>0.05) for OS. Transfusion dependency, a confounding factor, could reflect the more objective parameter of severity of anemia and had impact on the survival (p<0.05), but not on the AML evolution. In the MDS patients a small scale of study showed that red cells transfusion at diagnosis and the intensity may be an important independent prognostic factor.33 In most Chinese patients, the transfusion threshold for RBC was lower than 60 g/L, and the patients with Hb concentrations greater than 70 g/L had no symptoms related to anemia and did not require red cell transfusion. On the other hand, the threshold for RBC transfusion in the westerns countries is lower than 80 g/L. Therefore, the prognostic value of concentration threshold of 80 g/L may possibly explain the difference of the Hb for the prognosis of survival. We further assessed the WPSS for OS, and found that high and very high groups had significance for OS (p<0.001). This finding needs to be confirmed by more studies. In the present study, multivariate analysis showed that the patient with marrow blasts ≥5% combining the trisomy 8 abnormalities had poorer prognosis on OS (p<0.05) and AML evolution (p<0.05) than the patient with <5% marrow blasts, in accordance with the findings by Saumell, et al.34
The new proposals for cytogenetic categorization, like the previous IPSS classification, have regarded isolated trisomy 8 as an intermediate risk prognosis to MDS. The median OS reported for patients with single trisomy 8 ranged between 11 and 25 months, and for the patients in the intermediate IPSS subgroup between 23 and 32 months.561035 Our results are in accordance with the results reported by Saumell, et al.;34 in our study the median OS for patients with single trisomy 8 was 32.3 months. We found that the complexity of additional aberrations had impact on OS; the single tr8 between tr8+1 aberration (p=0.003), tr8+1 aberration between tr8+≥2 aberrations (p=0.001), and the median OS for tr8+1 and tr8+≥2 were 22.5 and 11.9 months respectively. This phenomenon was different from the results by Haase, et al.,35 who showed that tr8 plus one additional anomaly had a better prognosis and were classified into good-prognostic cytogenetic subgroup. However, our data demonstrated that there was statistical difference in median survival between the two subgroups. Similarly, the progression to AML risk increased with the complexity of the cytogenetic abnormalities, but the result must be confirmed with multicenter studies.
Compared with the IPSS, IPSS-R defined 16 specific abnormalities better, grouped into five different risk groups, and classified a number of uncommon cytogenetic subsets in accordance with its sophisticated stratification.9 In addition, recent evidence suggests that the prognostic significance of poor cytogenetics has been underestimated in the IPSS, while the defect has been improved in the IPSS-R prognostic models.36 We applied the IPSS-R to an independent group of 86 Chinese MDS patients with tr8 alterations, and evaluated the predictive power of the two systems for the OS and the risk of AML transformation. Two systems both could successfully discriminate risk groups for survival. The same cohort of patient distribution into risk groups varied according to the scoring systems: IPSS-R was more specific than IPSS for discriminating survival risk groups, but had no advantage in the prognosis for the risk of the AML transformation in this cohort. This result can be explained by the limits of the small number of enrolled patients, and need to be confirmed by more multicenter researches.
In summary, the results of this retrospective study confirmed the impact of the number of additional aberration on MDS patients with tr8, and first assessed the importance of clinical parameters for the outcomes. This series may be useful for the design of more clinical trials in Chinese MDS patients with tr8.
Figures and Tables
 | Fig. 1Survival according to WPSS. WPSS, World Health Organization Classification-Based Scoring System. |
 | Fig. 2Survival according to cytogenetic complexity. tr8, +8, trisomy 8; WPSS, World Health Organization Classification-Based Scoring System. |
 | Fig. 3Overall survival according to IPSS-R. IPSS-R, Revised International Prognostic System; WPSS, World Health Organization Classification-Based Scoring System. |
Table 1
Univariate Analysis of Prognostic Parameters for OS and AML Progression
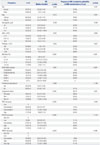
ANC, absolute neutrophil count; WHO, World Health Organization; RCUD, refractory cytopenia with unlineage dysplasia; RCMD, refractory cytopenia with multilineage dysplasia; RARS, refractory anemia with ring sideroblasts; RAEB, refractory anemia with excess blasts; MDS-U, myelodysplastic syndromes unclassifiable; tr8, +8, trisomy 8; IPSS, International Prognostic Scoring System; IPSS-R, Revised IPSS; WPSS, WHO Classification-Based Scoring System; OS, overall survival; AML, acute myeloid leukemia; BM, bone marrow.
Table 2
Cox Model for OS

ACKNOWLEDGEMENTS
In this study morphology examination by Xiao-Mei She and Lei Chen; data collection by Lei Chen, Bin Hu, and Qing-Fang Yue; analysis data by Qing-Fang Yue and Lei Chen; drafting of the manuscript by Qing-Fang Yue; critical revision of the manuscript for important intellectual content by Yu Hu, Ping Zou, and Xin-Yue Liu.
References
1. Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2002; 136–161.

3. Nolte F, Hofmann WK. Myelodysplastic syndromes: molecular pathogenesis and genomic changes. Ann Hematol. 2008; 87:777–795.

4. Mufti GJ, Bennett JM, Goasguen J, Bain BJ, Baumann I, Brunning R, et al. Diagnosis and classification of myelodysplastic syndrome: International Working Group on Morphology of myelodysplastic syndrome (IWGM-MDS) consensus proposals for the definition and enumeration of myeloblasts and ring sideroblasts. Haematologica. 2008; 93:1712–1717.

5. Solé F, Luño E, Sanzo C, Espinet B, Sanz GF, Cervera J, et al. Identification of novel cytogenetic markers with prognostic significance in a series of 968 patients with primary myelodysplastic syndromes. Haematologica. 2005; 90:1168–1178.
6. Bernasconi P, Klersy C, Boni M, Cavigliano PM, Calatroni S, Giardini I, et al. World Health Organization classification in combination with cytogenetic markers improves the prognostic stratification of patients with de novo primary myelodysplastic syndromes. Br J Haematol. 2007; 137:193–205.

7. Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007; 25:3503–3510.

8. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120:2454–2465.

10. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89:2079–2088.

11. Paulsson K, Johansson B. Trisomy 8 as the sole chromosomal aberration in acute myeloid leukemia and myelodysplastic syndromes. Pathol Biol (Paris). 2007; 55:37–48.

12. Irons RD, Wang X, Gross SA, Bao L, Ryder J, Chen Y, et al. Prevalence of MDS subtypes in Shanghai, China: a comparison of the World Health Organization and French American British classifications. Leuk Res. 2006; 30:769–775.

13. Qu S, Xu Z, Zhang Y, Qin T, Zhang T, Cui R, et al. Impacts of cytogenetic categories in the Revised International Prognostic Scoring System on the prognosis of primary myelodysplastic syndromes: results of a single-center study. Leuk Lymphoma. 2012; 53:940–946.

14. Xiao Y, Wei J, Chen Y, Zhang K, Zhou J, Zhang Y. Trisomy 8 is the most frequent cytogenetic abnormality in de novo myelodysplastic syndrome in China. Onkologie. 2012; 35:100–106.

15. Ma Y, Wang X, Xu X, Lin G. Prognostic value of trisomy 8 in primary myelodysplastic syndrome. Intern Med J. 2010; 40:697–703.

16. Cazzola M, Della Porta MG, Travaglino E, Malcovati L. Classification and prognostic evaluation of myelodysplastic syndromes. Semin Oncol. 2011; 38:627–634.

17. Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, Keyvanafar K, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005; 106:841–851.

18. Olnes MJ, Shenoy A, Weinstein B, Pfannes L, Loeliger K, Tucker Z, et al. Directed therapy for patients with myelodysplastic syndromes (MDS) by suppression of cyclin D1 with ON 01910.Na. Leuk Res. 2012; 36:982–989.

19. Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica. 2011; 96:1433–1440.

20. Gonzalez Garcia JR, Meza-Espinoza JP. Use of the International System for Human Cytogenetic Nomenclature (ISCN). Blood. 2006; 108:3952–3953.

21. Chun K, Hagemeijer A, Iqbal A, Slovak ML. Implementation of standardized international karyotype scoring practices is needed to provide uniform and systematic evaluation for patients with myelodysplastic syndrome using IPSS criteria: An International Working Group on MDS Cytogenetics Study. Leuk Res. 2010; 34:160–165.

22. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951.

23. Wellner JA. On an exponential bound for the Kaplan-Meier estimator. Lifetime Data Anal. 2007; 13:481–496.

25. Pozdnyakova O, Miron PM, Tang G, Walter O, Raza A, Woda B, et al. Cytogenetic abnormalities in a series of 1,029 patients with primary myelodysplastic syndromes: a report from the US with a focus on some undefined single chromosomal abnormalities. Cancer. 2008; 113:3331–3340.

26. Matsuda A, Germing U, Jinnai I, Misumi M, Kuendgen A, Knipp S, et al. Difference in clinical features between Japanese and German patients with refractory anemia in myelodysplastic syndromes. Blood. 2005; 106:2633–2640.

27. Lee JJ, Kim HJ, Chung IJ, Kim JS, Sohn SK, Kim BS, et al. Comparisons of prognostic scoring systems for myelodysplastic syndromes: a Korean multicenter study. Leuk Res. 1999; 23:425–432.

28. Kuendgen A, Matsuda A, Germing U. Differences in epidemiology of MDS between Western and Eastern countries: ethnic differences or environmental influence? Leuk Res. 2007; 31:103–104.

29. Chen B, Zhao WL, Jin J, Xue YQ, Cheng X, Chen XT, et al. Clinical and cytogenetic features of 508 Chinese patients with myelodysplastic syndrome and comparison with those in Western countries. Leukemia. 2005; 19:767–775.

30. Li L, Liu XP, Nie L, Yu MH, Zhang Y, Qin TJ, et al. Unique cytogenetic features of primary myelodysplastic syndromes in Chinese patients. Leuk Res. 2009; 33:1194–1198.

31. Al Ameri A, Jabbour E, Garcia-Manero G, O'Brien S, Faderl S, Ravandi F, et al. Significance of thrombocytopenia in myelodysplastic syndromes: associations and prognostic implications. Clin Lymphoma Myeloma Leuk. 2011; 11:237–241.

32. Lee JH, Lee JH, Shin YR, Lee JS, Kim WK, Chi HS, et al. Application of different prognostic scoring systems and comparison of the FAB and WHO classifications in Korean patients with myelodysplastic syndrome. Leukemia. 2003; 17:305–313.

33. Pereira A, Nomdedeu M, Aguilar JL, Belkaid M, Carrió A, Cobo F, et al. Transfusion intensity, not the cumulative red blood cell transfusion burden, determines the prognosis of patients with myelodysplastic syndrome on chronic transfusion support. Am J Hematol. 2011; 86:245–250.

34. Saumell S, Florensa L, Luño E, Sanzo C, Cañizo C, Hernández JM, et al. Prognostic value of trisomy 8 as a single anomaly and the influence of additional cytogenetic aberrations in primary myelodysplastic syndromes. Br J Haematol. 2012; 159:311–321.

35. Haase D, Germing U, Schanz J, Pfeilstöcker M, Nösslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007; 110:4385–4395.

36. Schanz J, Steidl C, Fonatsch C, Pfeilstöcker M, Nösslinger T, Tuechler H, et al. Coalesced multicentric analysis of 2,351 patients with myelodysplastic syndromes indicates an underestimation of poor-risk cytogenetics of myelodysplastic syndromes in the international prognostic scoring system. J Clin Oncol. 2011; 29:1963–1970.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download