Abstract
Purpose
The detection of high-level tetracycline-resistant strains of Neisseria gonorrhoeae (TRNG) can make important epidemiological contributions that are relevant to controlling infections from this pathogen. In this study, we aimed to determine the incidence of TRNG isolates over time and also to investigate the characteristics and genetic epidemiology of these TRNG isolates in Korea.
Materials and Methods
The antimicrobial susceptibilities of 601 isolates of N. gonorrhoeae from 2004 to 2011 were tested by standard Clinical and Laboratory Standards Institute methods. To determine the molecular epidemiological relatedness, N. gonorrhoeae multi-antigen sequence typing was performed.
Results
The incidence of TRNG increased from 2% in 2004 to 21% in 2011. The minimum inhibitory concentration distributions of ceftriaxone and susceptibility of ciprofloxacin in TRNG were different from non-TRNG and varied according to the year of isolation. Most of the TRNG isolates collected from 2004 to 2007 exhibited genetic relatedness, with sequence type (ST) 1798 being the most common. From 2008 to 2011, the STs of the isolates became more variable and introduction of genetically unrelated TRNG were noted.
Neisseria gonorrhoeae (N. gonorrhoeae) is the causative agent of gonorrhea, a sexually transmitted disease, and is one of the most prevalent sexually-transmitted pathogens worldwide.1 The incidence of N. gonorrhoeae infection has decreased in many developed countries,2 although it is still prevalent in many developing counties,1 and has even recently increased in a few developed countries.3 According to the Centers for Disease Control and Prevention (CDC) in the United States, a total of 321849 cases of gonococcal infections were reported in 2011; however, the CDC estimates that the incidence could exceed 800000 cases annually in the United States alone.4 In Korea, the Health Insurance Review and Assessment Service reported that 40038 cases of gonococcal infections were charged for pay in 2012.5
N. gonorrhoeae is considered to be a pathogen, and can only be transmitted from human to human via various sexual practices.6 Thus, infection control is very important to prevent the dissemination of N. gonorrhoeae among the general population. However, most female and a few male patients who have gonorrhea do not exhibit any apparent symptoms,7 despite the clinical significance of this disease. The lack of obvious symptoms therefore adds a layer of difficulty to controlling its spread. Furthermore, the emergence and spread of resistance to various antimicrobial agents has created many difficulties in the management of gonococcal infections.89 Drug-resistant N. gonorrhoeae was classified as a microorganism with a threat level of "urgent" by the CDC in 2013.4 Thus, epidemiological understanding and surveillance programs may need to be implemented in order to prevent the dissemination of gonorrhea and overcome the problem of antimicrobial resistance in N. gonorrhoeae.10
Tetracycline is a historic drug and cannot be used any longer to treat gonorrhea in many countries due to the dissemination of tetracycline-resistant strains of N. gonorrhoeae, which have become very common over the past several decades, including in Korea.11 Since tetracycline is not used anymore, the detection of tetracycline resistance is clinically insignificant. However, surveillance of trends in high-level resistance to tetracycline caused by plasmid transfer can be helpful in understanding the epidemiological aspects of gonococcal infections and controlling these infections, because such monitoring enables the detection of clonal variations.12 In this study, we aimed to determine the incidence of high-level tetracyclineresistant N. gonorrhoeae (TRNG) isolates over many years and to investigate the characteristics and genetic epidemiology of TRNG in Korea.
A total of 601 N. gonorrhoeae isolates were collected from symptomatic patients with urethritis and sex workers between 2004 and 2011 in Korea. The number of isolates collected each year ranged from 47 to 135. Most N. gonorrhoeae isolates were obtained from primary urological clinics, which were widely distributed across the country. Modified Thayer-Martin agar plates (Becton Dickinson, Cockeysville, MD, USA) were used for primary isolation, and conventional biochemical tests, including Gram stain and the Vitek NHI System (bioMerieux, Marcy l'Etoile, France), were used for species identification. Isolates were stored in 20 per cent skim milk (Difco, Detroit, MI, USA) at -70℃ until ready for analysis.
High-level resistance to tetracycline was tested by the disk diffusion and agar dilution methods. The disk diffusion test, which is recommended by the Clinical and Laboratory Standards Institute (CLSI), was performed using a disk containing 30 µg of tetracycline (Becton Dickinson).1314 Fifty-eight isolates of TRNG and 304 isolates of non-TRNG were randomly selected to determine the minimum inhibitory concentrations (MICs) of various antimicrobial agents, including tetracycline, and to compare resistances. Resistance to penicillin G (Sigma Chemical, Saint Louis, MO, USA), ceftriaxone (Hanmi, Seoul, Korea), spectinomycin (Kuk Je, Seoul, Korea), cefixime (Dong-A, Seoul, Korea), tetracycline (Pfizer Korea, Seoul, Korea), and ciprofloxacin (Bayer Korea, Seoul, Korea) were tested by the CLSI agar dilution method,1315 using a GC II agar base supplemented with 1% IsoVitaleX (Becton Dickinson). Approximately 104 colony forming unit of each isolate were inoculated onto the agar plates with a Steer's replicator (Craft Machine, Chester, PA, USA); plates were then incubated in a 5% CO2 atmosphere at 35℃ for 24 h. The American Type Culture Collection (ATCC) 49226, World Health Organization (WHO) A 100A6, WHO B 100A7, WHO G 100I1, WHO J 100I4, WHO K 300J4, and WHO L 00G 1003 OKKA20 strains of N. gonorrhoeae were used as quality control strains.
To determine the molecular epidemiological relatedness of the TRNG strains, N. gonorrhoeae multi-antigen sequence typing (NG-MAST) and pulsed-field gel electrophoresis (PFGE) were carried out for 58 and 36 isolates of TRNG, and NG-MAST for 156 isolates of non-TRNG was tested.12 NG-MAST was performed according to the guidelines outlined on the NG-MAST homepage (http://www.ng-mast.net), using primers for the porB and tbpB genes.16 Polymerase chain reaction (PCR) was carried out in a total volume of 20 µL, containing 1 µL of heatextracted template DNA, 10 pmol of each primer, and a PreMix (Bioneer, Daejeon, Korea) master mix containing 1 U of Taq DNA polymerase. Thermocycling conditions included 25 cycles of 95℃ for 30 seconds, either 58℃ for 30 seconds for porB or 69℃ for 30 seconds for tbpB, and 72℃ for 1 minute. Reactions also included an initial denaturation step at 95℃ for 4 minutes and a final extension step at 72℃ for 10 minutes, and were performed using a thermal cycler (Eppendorf, Hamburg, Germany). The resultant PCR products were purified using a DNA extraction kit (Qiagen, Hilden, Germany), and the nucleotide sequences of the PCR-generated amplicons were analyzed at a commercial laboratory (Macrogen, Seoul, Korea). The sequences of porB and tbpB were analyzed using the NG-MAST homepage to assign allele numbers and to determine the sequence type (ST) of each isolate. Two phylogenetic trees of por genes for TRNG and non-TRNG and tbpB genes for TRNG were generated with Molecular Evolutionary Genetics Analysis version 6 software (http://www.megasoftware.net) using the Neighbor-Joining method.
PFGE was performed for 36 isolates, which showed different NG-MAST STs. Briefly, one loopful of cells was suspended in 1 mL of saline ethylenediaminetetraacetic acid (EDTA) solution and used to prepare genomic DNA plugs, which were digested with NheI (Takara, Tokyo, Japan) for 18 hours at 35℃. Fragments were then resolved using switch times of 0.5 seconds (initial) and 54 s (final), and a running time of 20 h at 6 V/cm with a CHEF DR II instrument (Bio-Rad, Hercules, CA, USA). PFGE banding pattern similarities were determined using the Dice coefficients and the unweighted pair group method, using arithmetic averages obtained by the clustering method. Calculations were performed using Molecular Analyst Fingerprinting software (v. 1.12; Bio-Rad), and dendrogram was generated from these calculations.
A total of 92 isolates (15%) of TRNG were identified from 2004 to 2011. The proportion of TRNG was low in 2004 (2%, 2/91); however, it increased to 10% (5/48) in 2005. This incidence of TRNG continued to steadily increase, reaching 24% (16/66) in 2007. The range of the incidence of TRNG from 2008 to 2011 was 15–21% (Fig. 1).
None of the non-TRNG isolates were susceptible to penicillin G; however, all of the non-TRNG isolates showed susceptibility to ceftriaxone, cefixime, and spectinomycin. The MIC50 and MIC90 of ceftriaxone and cefixime were 0.06 mg/L and 0.12 mg/L, respectively. The MIC50 and MIC90 of ciprofloxacin were 8 mg/L and 16 mg/L, and 15% of all isolates were susceptible to ciprofloxacin. Like the non-TRNG isolates, none of the TRNG isolates were susceptible to penicillin G; meanwhile, all TRNG isolates were susceptible to ceftriaxone, cefixime, and spectinomycin. However, the MIC50s and MIC90s of ceftriaxone and cefixime were very low (≤0.008 mg/L), unlike for the non-TRNG isolates. The rate of susceptibility to ciprofloxacin among the TRNG isolates was 61%, and the MIC50 and MIC90 of ciprofloxacin were ≤0.008 mg/L and 4 mg/L, respectively (Table 1).
From 2004 to 2011, no definite changes in antimicrobial susceptibility were noted in the non-TRNG isolates; however, the susceptibility to cephalosporins and the rate of susceptibility to ciprofloxacin deteriorated in the TRNG isolates. The MIC50 and MIC90 of ceftriaxone were ≤0.008 mg/L and ≤0.008 mg/L in 2004–2007, ≤0.008 mg/L and 0.06 mg/L in 2008–2009, and 0.03 mg/L and 0.06 mg/L in 2010–2011, respectively. The MIC50 of ciprofloxacin and rate of susceptibility were ≤0.008 mg/L and 93% in 2004–2007, 2 mg/L and 44% in 2008–2009, and 4 mg/L and 18% in 2010–2011, respectively (Table 1).
From 2004 to 2007, five different STs were found in 12 isolates; of these, six isolates belonged to ST1798 (50%) according to NG-MAST analysis, and nine isolates contained the tbpB 455 allele. All isolates collected in this period were highly susceptible to both ceftriaxone and cefixime, and all isolates except one showed susceptibility to ciprofloxacin. During 2008 and 2009, the STs of the isolates became more variable, with 13 different STs observed in 24 isolates; however, ST1798 remained the most prominent ST (7/24). Most of the N. gonorrhoeae isolates harboring the tbpB 455 allele showed susceptibility to ciprofloxacin, but other isolates that harbored the tbpB 21, 33, 60, 66, 110, or 272 allele were either intermediately or completely resistant. During 2010 and 2011, 15 different STs were noted; moreover, no two isolates shared the same ST. The most common tbpB allele was 21 (7/15), and only three isolates harbored the tbpB 455 allele, even though this allele had been highly prevalent during previous periods. Most of the isolates were resistant to ciprofloxacin, except the ST7934 and ST7924 isolates (Table 2). Dendrogram analysis revealed that most isolates were genetically unrelated, and suggested the sporadic emergence of various STs from 2007 to 2011. Some isolates harboring the same tbpB allele belonged to various STs, which were located distantly from one another on the dendrogram of TRNG (data not shown). In the phylogenetic tree, which was composed for the por gene for TRNG and non-TRNG isolates in 2006, three TRNG isolates had por 1146, while another exhibited por 2392; both alleles were genetically related to each other. However, the por gene of TRNG varied during 2010 and 2011, and the location of por gene alleles were dispersed throughout the phylogenetic tree (Fig. 2). In the phylogenetic tree of tbpB genes, which was composed for the 11 tbpB alleles identified from 2004 to 2011, two clusters were noted. The large cluster consisted of tbpB 33, 60, 110, 129, 330, 455, and 860, whereas the smaller cluster comprised tbpB 4, 21, 66, and 272 (Fig. 3).
The dissemination of multidrug-resistant strains of N. gonorrhoeae and the recent emergence of resistance to extendedspectrum cephalosporins, including ceftriaxone, pose major problems for the management of gonorrhea.171819 A particular difficulty in achieving effective antimicrobial treatment is that it requires active infection control, based on precise epidemiological information, to overcome gonorrheal infections. Tetracycline resistance can be caused by chromosomal mutations in several genes, such as mtrR, or by the transfer of plasmids harboring the tetM gene.2021
In Korea, tetracycline resistance has been widely disseminated since the late 1960s, and almost all isolates have been tetracycline-resistant for several decades.112223 However, in spite of the prevalence of tetracycline resistance, the incidence of TRNG remained relatively low until the early 2000s, ranging from 0–1% in the late 1990's and 1–4% in the early 2000's.1222 In 2004, the incidence of TRNG was still low (2%) in Korea; however, high-level tetracycline resistance increased to 10% in 2005 and again up to 24% in 2007. The reasons underlying this increase in TRNG isolates are not yet clear. However, hypotheses can be generated by taking into account clonal spread, horizontal spread, and foreign import. First, clonal spread may have contributed to the rise of TRNG in Korea. In antimicrobial susceptibility comparisons, TRNG isolates showed differences in their cephalosporin and ciprofloxacin susceptibilities, compared with non-TRNG isolates. The MIC50s and MIC90s of ceftriaxone and cefixime for TRNG isolates were eight times lower than for non-TRNG isolates; furthermore, the rate of ciprofloxacin susceptibility was 61% in TRNG isolates, but only 15% in non-TRNG isolates. This difference can be interpreted in the context of clonal differences and expansion of TRNG clones; moreover, clonal spread of TRNG isolates has been observed in the other countries including UK and Argentina.2425 The importance of clonal dissemination is also supported by the observation that most of the TRNG isolates obtained from 2004 to 2007 were of the ST1798 subtype and harbored the tbpB 455 allele (ST1798, ST6327, and ST7927); moreover, these isolates were placed into one common phylogenic cluster, shared with the tbpB 33 (ST7894) and tbpB 860 (ST3945) alleles.
However, the susceptibilities of TRNG isolates to ceftriaxone and ciprofloxacin were not consistent, and decreased over time. From 2004 to 2007, the MIC50s and MIC90s of TRNG isolates to ceftriaxone and cefixime were lower than 0.008 mg/L. From 2008 to 2009, the MIC90s of ceftriaxone and cefixime increased to 0.06 mg/L; moreover, the MIC50s of ceftriaxone and cefixime also increased to 0.03 mg/L and 0.06 mg/L, respectively. The MIC50 of ciprofloxacin and the rate of resistance to ciprofloxacin also increased over time. These changes in the antimicrobial susceptibilities of TRNG isolates were also reported in India by Bala, et al.26 Such changes can result from the horizontal spread of a plasmid bearing the tetM gene to resistant non-TRNG isolates, because plasmids can move between isolates.272829 This phenomenon may provide a second explanation for the increase in TRNG isolates. However, considering the fact that the selective pressure for tetracycline was very low in Korea since the 1990s,22 horizontal transfer seems a less likely explanation than the other alternatives.
Notwithstanding, many isolates of TRNG were located at a great distance from one another in the dendrogram and phylogenetic tree in the period of increasing prevalence of TRNG. In 2006, three of four TRNG isolates belonged to ST1798 harboring por 1146 allele, while the remaining isolate exhibited por 2392, revealing a genetic relationship indicative of clonal expansion of TRNG. However, this clonal relationship was not found during 2010 and 2011. TRNG collected during this period seemed to appear sporadically, and their appearance is not easily explained by clonal spread. During this period, half of the por alleles (8/16) were located outside of the main cluster that consisted of por 23, 2053, 2874, 4270, 4801, 4917, 6288, and 6297, which may indicate emergence of divergent TRNG strains. The emergence of these divergent isolates can suppose that the increase of TRNG prevalence can result from foreign import when we take into consideration that consistent collection strategies have been sustained since 2004. This hypothesis also can be supported by a change in the tbpB allele throughout the late 2000s in Korea. From 2004 to 2007, the majority of all TRNG isolates in Korea belonged to STs bearing the tbpB 455 allele (ST1798, ST6327, and ST7927); however, STs harboring the tbpB 21 allele (ST7689 and ST7928) emerged in 2008, making this allele the most prominent allele during 2010 and 2011. Strains harboring the tbpB 455 or tbpB 21 allele showed many differences in their individual allele sequences, and this can suggest the emergence of new STs resulting from foreign import.30 In the Asia-Pacific region, the proportion of TRNG isolates varies according to country.3132 In 2004, 72% of all N. gonorrhoeae isolates in Singapore were TRNG, 34% in China, 20% in Vietnam and 8% in the Philippines. Japan exhibited a low incidence (2%) of TRNG, similar to that of Korea.31 In 2010, the incidence of TRNG isolates in Western-Pacific countries ranged from moderate to high, with incidences of 71–100% in Brunei; 35–75% in Mongolia, China, Hong Kong, Singapore, and Vietnam; and 10–34% in Australia, India, the Philippines, and Thailand.32 It is difficult to thoroughly assess the extent of foreign import of some pathogens; however, the recent worldwide spread of cefixime-resistant strains of N. gonorrhoeae33 and genetic relatedness of ceftriaxone-resistant strains isolated from France and Spain may be a convincing examples of foreign import.34 In our study, the emergence of new STs, the changes in tbpB allele distribution, the dispersed distribution of isolates sharing the same tpbB allele in the dendrogram, and the dispersed distribution of isolates of various STs according to year in the dendrogram appear to support the idea of foreign import. This import of TRNG strains from other countries can be a source of another severe antimicrobial resistance, including ceftriaxone resistance, and may require additional programs to prevent it. However, further investigations employing additional approaches, such as multilocus sequence typing, should be performed to test this hypothesis.
In conclusion, the increase in the incidence of TRNG strains until 2007 appears to be due, at least in part, to clonal spread. However, we propose that the emergence of various STs since 2008 is due to foreign import and/or clonal evolution. Further investigations are required to fully determine the relevant changes in TRNG epidemiology.
Figures and Tables
 | Fig. 1Summary of the number and percentage of TRNG isolates obtained in Korea by year. TRNG, high-level tetracycline-resistant Neisseria gonorrhoeae. |
 | Fig. 2Evolutionary relationships of por gene of TRNG and non-TRNG isolated in Korea during 2006 (A) and 2010–2011 (B). The phylogenetic tree was drawn by MEGA6 software using the Neighbor-Joining method. Allele numbers belonging to TRNG are marked with a red dot. TRNG, high-level tetracycline-resistnat Neisseria gonorhoeae; MEGA6, Molecular Evolutionary Genetics Analysis version 6. |
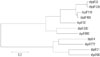 | Fig. 3Phylogenetic tree of the tbpB alleles harbored by various TRNG isolates obtained in Korea from 2004 to 2011 made by MEGA6 software using the Neighbor-Joining method. TRNG, high-level tetracycline-resistnat Neisseria gonorhoeae; MEGA6, Molecular Evolutionary Genetics Analysis version 6. |
Table 1
Comparison of the Antimicrobial Susceptibilities of TRNG and Non-TRNG Isolates during 2004–2007, 2008–2009, and 2010–2011

Table 2
NG-MAST Sequence Types of TRNG Isolates Obtained from 2004 to 2011

ACKNOWLEDGEMENTS
The authors are grateful to Ms. Hayeon Kim for collecting the isolates. This work was supported by the Korean Centers for Disease Control and Prevention as one of project of national surveillance program supported by Korean Centers for Disease Control and Preventions (2012-E44006-00).
The work was performed at the Department of Laboratory Medicine, Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea.
References
1. WHO. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. accessed on 2015 Jan 5. Available at: http://www.who.int/reproductivehealth/publications/rtis/2008_STI_estimates.pdf.
2. Unemo M, Ison CA, Cole M, Spiteri G, van de Laar M, Khotenashvili L. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex Transm Infect. 2013; 89:Suppl 4. iv42–iv46.

3. Centers for Disease Control and Prevention. Transmitted Disease Surveillance 2012. accessed on 2015 Jan 5. Available at: http://www.cdc.gov/sTD/stats12/Surv2012.pdf.
4. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. accessed on 2015 Jan 5. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
5. Health Insurance Review & Assessment Service. Statistics for Diseases and Treatment in Korea. accessed on 2015 Jan 5. Available at: http://www.hira.or.kr/rd/dissdic/infoSickList.do?pgmid=HIRAA020044020100.
6. Elias J, Frosch M, Vogel U. Neisseria. In : Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th ed. Washington, DC: ASM Press;2011. p. 559–573.
7. Chacko MR, Wiemann CM, Smith PB. Chlamydia and gonorrhea screening in asymptomatic young women. J Pediatr Adolesc Gynecol. 2004; 17:169–178.

8. Barbee LA. Preparing for an era of untreatable gonorrhea. Curr Opin Infect Dis. 2014; 27:282–287.

9. Unemo M, Golparian D, Shafer WM. Challenges with gonorrhea in the era of multi-drug and extensively drug resistance - are we on the right track? Expert Rev Anti Infect Ther. 2014; 12:653–656.

10. WHO. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. accessed on 2015 Jan 5. Available at: http://apps.who.int/iris/bitstream/10665/44863/1/9789241503501_eng.pdf.
11. Chong Y, Kim SO, Yi KN, Lee SY. Penicillin and tetracycline susceptibility of Neisseria gonorrhoeae strains isolated during 1966 to 1975. Yonsei Med J. 1976; 17:46–51.

12. Lee K, Shin JW, Lim JB, Kim YA, Yong D, Oh HB, et al. Emerging antimicrobial resistance, plasmid profile and pulsed-field gel electrophoresis pattern of the endonuclease-digested genomic DNA of Neisseria gonorrhoeae. Yonsei Med J. 2000; 41:381–386.

13. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute;2013.
14. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2012.
15. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2012.
16. Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004; 189:1497–1505.

17. Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, et al. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002; 46:3744–3749.

18. Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011; 17:148–149.
19. Wright DJ, Azadian B. Cephalosporin resistance in gonorrhoea. Lancet Infect Dis. 2013; 13:728–730.

20. Sparling PF, Sarubbi FA Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975; 124:740–749.

21. Morse SA, Johnson SR, Biddle JW, Roberts MC. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986; 30:664–670.

22. Lee H, Hong SG, Soe Y, Yong D, Jeong SH, Lee K, et al. Trends in antimicrobial resistance of Neisseria gonorrhoeae isolated from Korean patients from 2000 to 2006. Sex Transm Dis. 2011; 38:1082–1086.

23. Kim HJ, Seo Y, Kim WH, Lee Y, Lee H, Lee K, et al. Antimicrobial resistance and molecular epidemiologic characteristics of Neisseria gonorrhoeae isolated from Korea in 2013. Ann Clin Microbiol. 2013; 16:182–187.

24. Palmer HM, Young H. Dramatic increase in a single genotype of TRNG ciprofloxacin-resistant Neisseria gonorrhoeae isolates in men who have sex with men. Int J STD AIDS. 2006; 17:254–256.

25. Fernandez Cobo M, Galarza P, Sparo M, Buscemi L, Pizarro MR, Fiorito S. Characterization of an outbreak of tetM-containing Neisseria gonorrhoeae in Argentina. Int J STD AIDS. 1999; 10:169–173.

26. Bala M, Jain RK, Ray K. Antimicrobial susceptibility profile of resistance phenotypes of Neisseria gonorrheae in India. Sex Transm Dis. 2008; 35:588–591.

27. Roberts MC, Knapp JS. Host range of the conjugative 25.2-megadalton tetracycline resistance plasmid from Neisseria gonorrhoeae and related species. Antimicrob Agents Chemother. 1988; 32:488–491.

28. Greco V, Ng LK, Catana R, Li H, Dillon JA. Molecular epidemiology of Neisseria gonorrheae isolates with plasmid-mediated tetracycline resistance in Canada: temporal and geographical trends (1986-1997). Microb Drug Resist. 2003; 9:353–360.

29. Moodley P, Hoppenbrouwers J, Bohlken L, Sturm AW. Emergence of TetM-mediated tetracycline resistance in rural South Africa. J Antimicrob Chemother. 2001; 48:142–143.

30. Olsen B, Hadad R, Fredlund H, Unemo M. The Neisseria gonorrhoeae population in Sweden during 2005-phenotypes, genotypes and antibiotic resistance. APMIS. 2008; 116:181–189.

31. WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific Region, 2004. Commun Dis Intell Q Rep. 2006; 30:129–132.
32. Lahra MM. WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2010. Commun Dis Intell Q Rep. 2012; 36:95–100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download