Abstract
Purpose
This study compared the angiographic outcomes of paclitaxel-coated balloon (PCB) versus plain old balloon angioplasty (POBA) treatment for de novo coronary artery lesions. At present, there is no available data comparing the efficacy of PCB versus POBA for the treatment of de novo coronary lesions.
Materials and Methods
This multicenter retrospective observational study enrolled patients with de novo coronary lesions with a reference vessel diameter between 2.5 mm and 3.0 mm and lesion length ≤24 mm who were successfully treated with PCB or POBA. Angiographic measurements and quantitative coronary analysis were performed before and after the procedure, and at 9 months follow-up.
Results
A total of 72 patients (49 receiving PCB and 23 receiving POBA) were enrolled in this study. Late luminal loss was -0.12±0.30 mm in the PCB group and 0.25±0.50 mm in the POBA group (p<0.001). There was a higher percentage of binary restenosis (diameter stenosis ≥50%) in POBA, compared to PCB (30.4%, n=7 vs. 4.1%, n=2, p<0.001). Target vessel revascularization was higher in the POBA group (13.0%, n=3 vs. 0%, p=0.033).
Although the conventional treatment for de novo coronary lesions is drug-eluting stent (DES) implantation, plain old balloon angioplasty (POBA) is still useful for patients unable to tolerate prolonged dual antiplatelet therapy or anatomically difficult lesions in small sized coronary vessels where stenting is impossible.12 Unfortunately, POBA has important limitations, including poor vessel patency, high restenosis rates due to elastic recoil, and late negative remodeling.3 The application of a balloon with anti-proliferative coating can overcome some of these deficiencies by preventing restenosis caused by neointimal hyperplasia. In this regard, paclitaxel-coated balloon (PCB) treatment is an attractive therapeutic option and may have benefits over POBA.4 The advantages of PCB include a homogeneous drug delivery to the vessel wall, an immediate drug release without the use of a polymer, the potential of reducing the intensity and duration of antiplatelet therapy and the freedom of leaving no foreign object behind in the vessel.5
The effects of treatment of de novo coronary lesions with PCB in comparison to POBA have not been previously investigated. Accordingly, the aim of this study was to compare angiographic outcomes between PCB treatment and POBA in de novo coronary lesions using quantitative coronary analysis
(QCA).
This multicenter retrospective observational study enrolled patients treated successfully with PCB and POBA between June 2010 and December 2013 from three teaching hospitals in South Korea. Patients with stable or unstable angina pectoris who were scheduled to undergo percutaneous coronary intervention (PCI) for de novo coronary lesions were enrolled if they had lesions with a ≥70% diameter stenosis, a reference vessel diameter of between 2.5 mm and 3.0 mm, and a lesion length of ≤24 mm. We retrospectively reviewed angiographic and clinical outcomes of enrolled patients at 9 months follow-up. Successful PCB and POBA treatments of de novo coronary lesions were defined by angiographic, procedural, and clinical criteria.6 Angiographic success of the procedure was considered as residual luminal narrowing in the dilated segment of <50% immediately after the procedure in the presence of thrombolysis in myocardial infarction flow grade 3.7 Procedural success was defined as angiographic success without major clinical complications (e.g., death, myocardial infarction, emergency coronary artery bypass surgery) during hospitalization.8 A clinically successful procedure was defined as anatomic and procedural success with relief of signs and/or symptoms of myocardial ischemia after the patient recovered from the procedure until discharge.6 Exclusion criteria included left ventricular ejection fraction of <30%, left main disease, heavily calcified or thrombotic lesions, life expectancy <1 year, and known chronic kidney disease (creatinine >2 mg/dL). Target lesion revascularization (TLR) was defined as any clinically driven repeat revascularization caused by a >50% stenosis within the POBA or PCB site or within a 5-mm border proximal or distal to the POBA or PCB site. Target vessel revascularization (TVR) was defined as any clinically driven repeat PCI of any segment within the entire epicardial coronary artery containing the target lesion. This study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board at Ulsan University Hospital. All enrolled patients provided written informed consent.
All patients were treated with acetylsalicylic acid 200 mg and a loading dose of clopidogrel 300 mg before the procedure, followed by maintenance clopidogrel 75 mg daily for 6 weeks and for extended periods thereafter at the physician's discretion. After obtaining coronary angiograms, patients underwent sequential pre-dilation with standard compliant or non-compliant balloons with a 1:1 balloon-to-vessel ratio and inflation at nominal pressure. For PCB treatment, the standard balloon was shorter than the intended PCB size, and the PCB (SeQuent Please®, PCB catheter, B. Braun, Melsungen, Germany) was inflated at nominal pressure for 60 seconds. Post-dilation was not performed in PCB or POBA cases. Coronary angiographies before and after the procedure and at 9 months follow-up were analyzed using the Cardiovascular Angiography Analysis System (CAAS 5.10, Pie Medical Imaging B.V., Maastricht, the Netherlands) by an independent investigator, who was blinded to clinical presentations.
All statistical analyses were conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistical methods were used to describe the data. Results are presented as mean±standard deviation for continuous variables and frequency (percentages) for categorical variables. Comparisons between the two groups were performed using an unpaired t-test for continuous variables and Pearson χ2 test for categorical variables. All tests were two-sided, and a p-value <0.05 was considered statistically significant.
In total, 72 patients (74 de novo lesions) were successfully treated with PCB (49 patients, 49 lesions) and POBA (23 patients, 25 lesions). Baseline clinical and procedural characteristics of the patients are shown in Table 1. A larger balloon diameter was used in the PCB group, compared to the POBA group (2.73±0.47 mm vs. 2.37±0.51 mm, p=0.021); however, there were no group differences in balloon to artery ratio (PCB group, 1.15±0.13 vs. POBA group, 1.18±0.23, p=0.627).
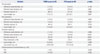
The QCA and clinical outcomes are shown in Table 2. Negative late luminal loss (LLL) was observed in the PCB group at 9 months follow-up. The in-segment LLL at 9 months was significantly lower in the PCB group than the POBA group (-0.12±0.30 mm vs. 0.25±0.50 mm, p<0.001). At 9 months, there was a higher percentage of binary restenosis (diameter stenosis ≥50%) in the POBA group (30.4%, n=7 vs. 4.1%, n=2, p<0.001). The clinical events observed were TLR and TVR, which occurred only in the POBA group.
The results of this study demonstrate the superiority of PCB treatment for de novo coronary lesions in suppressing neointimal hyperplasia in comparison to POBA. In fact, the use of the PCB resulted in a negative LLL, compared to POBA treatment alone, with an increase in minimal lumen diameter (MLD) in more than half of the patients (35 of 49) during follow-up.
Historically, remodeling and compression of an atherosclerotic plaque were thought to constitute the major mechanism of balloon angioplasty.9 However, previous studies have shown that nearly 50% of the theoretically achievable cross-sectional area is lost after balloon angioplasty, because of the elastic properties of the vessel and intimal hyperplasia.91011 Coronary stents were developed in part to overcome the risk of elastic recoil and restenosis from balloon angioplasty. Despite the clear benefits of coronary stents, alternatives are occasionally required for patients with de novo lesions requiring revascularization who are unable to tolerate long-term dual antiplatelet therapy due to high bleeding risk, poor compliance, or pending non-cardiac surgery, or where coronary anatomy prevents stent deployment.12 In these scenarios, PCB treatment as an adjunct to POBA provides an effective and safe alternative to coronary stent implantation.
Recent data suggests PCB treatment as an adjunct to POBA is feasible in patients with de novo coronary lesions.1213 The Valentines II trial demonstrated that PCB achieves high procedural success rates (99%) with acceptable rates of bail-out stenting (12%) and low adverse cardiac events rates at midterm follow-up (8.7%), and offered an alternative for revascularization in patients unsuitable for DES implantation.12 Another study showed that PCB treatment in de novo coronary arteries after pre-dilatation without major dissection and recoil led to late lumen increase (1.75±0.55 mm vs. 1.91±0.55 mm, p<0.001).13 In the same context, our study showed that PCB treatment, unlike POBA, achieves lumen increase in de novo coronary lesions at 9 months follow-up, although both PCB and POBA equally achieved procedural success upon completion of the procedure.
Although the exact mechanism of the late lumen increase is not well understood, this is thought to be likely due to the local drug delivery effects of paclitaxel. The sustained pharmacological effects of paclitaxel are exerted by binding to the subunit of tubulin, resulting in arrest of microtubule function, up-regulation of pro-apoptotic factors, and the promotion of prolonged antiproliferation.1415 Preceding laboratory results have shown that even a short contact between taxane compounds and vascular smooth muscle cells can inhibit the proliferation of the cells for a long period.1617 A previous study showed that the most pronounced lumen enlargement is seen in areas with the highest plaque burden.13 However, plaque regression or other healing mechanisms cannot be excluded without assessment with optical coherence tomography or intravascular ultrasound.
There are some limitations to our study that need consideration. Firstly, this study was a retrospective observational clinical study with small numbers. Secondly, the patients selected had relatively small coronary vessels; however, this is the current indication for PCB reimbursement in Korea. Although a further study is needed to evaluate these findings in larger arteries after PCB treatment, ethical considerations may make such a study hard to undertake, because of the clear benefits of coronary stents in large vessels amongst patients with no other contraindications. Finally, post-procedure reference vessel diameter and MLD were smaller in the POBA treatment group than the PCB treatment group. These two parameters may have affected the binary restenosis and TLR.
In conclusion, PCB treatment of de novo coronary lesions showed better angiographic outcomes at 9 months after the procedure than POBA treatment alone.
Figures and Tables
Table 1
Baseline Clinical and Procedural Characteristics
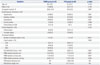
POBA, plain old balloon angioplasty; PCB, paclitaxel-coated balloon; LV, left ventricular; CAD, coronary artery disease; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; NA, not available; ACC, American College of Cardiology; AHA, American Heart Association.
Data are mean±standard deviation or number (percentage).
Table 2
Pre-Procedure, Post-Procedure, and 9-Month Angiographic Follow-Up Quantitative Coronary Analysis and the Clinical Events at 9 Months Follow-Up
References
1. Brilakis ES, Banerjee S, Berger PB. Perioperative management of patients with coronary stents. J Am Coll Cardiol. 2007; 49:2145–2150.

2. Agostoni P, Biondi-Zoccai GG, Gasparini GL, Anselmi M, Morando G, Turri M, et al. Is bare-metal stenting superior to balloon angioplasty for small vessel coronary artery disease? Evidence from a meta-analysis of randomized trials. Eur Heart J. 2005; 26:881–889.

3. Mintz GS. Remodeling and restenosis: observations from serial intravascular ultrasound studies. Curr Interv Cardiol Rep. 2000; 2:316–325.
4. Vos NS, Dirksen MT, Vink MA, van Nooijen FC, Amoroso G, Herrman JP, et al. Safety and feasibility of a PAclitaxel-eluting balloon angioplasty in Primary Percutaneous coronary intervention in Amsterdam (PAPPA): one-year clinical outcome of a pilot study. EuroIntervention. 2014; 10:584–590.

5. Kleber FX, Rittger H, Bonaventura K, Zeymer U, Wöhrle J, Jeger R, et al. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol. 2013; 102:785–797.

6. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006; 47:e1–e121.
7. Smith SC Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty). J Am Coll Cardiol. 2001; 37:2215–2239.
8. Kent KM, Bentivoglio LG, Block PC, Cowley MJ, Dorros G, Gosselin AJ, et al. Percutaneous transluminal coronary angioplasty: report from the Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1982; 49:2011–2020.

9. Haude M, Erbel R, Issa H, Meyer J. Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J Am Coll Cardiol. 1993; 21:26–34.

10. Rensing BJ, Hermans WR, Strauss BH, Serruys PW. Regional differences in elastic recoil after percutaneous transluminal coronary angioplasty: a quantitative angiographic study. J Am Coll Cardiol. 1991; 17:6 Suppl B. 34B–38B.

11. Liu MW, Roubin GS, King SB 3rd. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989; 79:1374–1387.

12. Waksman R, Serra A, Loh JP, Malik FT, Torguson R, Stahnke S, et al. Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention. 2013; 9:613–619.

13. Kleber FX, Schulz A, Waliszewski M, Hauschild T, Böhm M, Dietz U, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. 2015; 104:217–225.

14. Pires NM, Eefting D, de Vries MR, Quax PH, Jukema JW. Sirolimus and paclitaxel provoke different vascular pathological responses after local delivery in a murine model for restenosis on underlying atherosclerotic arteries. Heart. 2007; 93:922–927.

15. Gray WA, Granada JF. Drug-coated balloons for the prevention of vascular restenosis. Circulation. 2010; 121:2672–2680.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download