Abstract
We describe herein a case of an impending corneal perforation with a large descemetocele in a patient with previous penetrating keratoplasty (PKP) that subsequently was treated with an emergent lamellar keratoplasty using frozen preserved cornea. A 76-year-old male patient, who had a PKP, presented with a completely whitish and edematous graft accompanied by large epithelial defects. Although antibiotics and antiviral agents were tried for three days, the corneal stroma abruptly melted, except for the Descemet's membrane and endothelium. Cryopreserved corneal tissue that was kept at -80℃ was thawed and sutured on top of the remaining Descemet's membrane and endothelium. Pathological and microbiological tests were conducted using the remaining donor and recipient corneal tissues. After tectonic corneal transplantation on top of a large descemetocele, a healthy graft and relatively clear interfaces between graft-host junctions were maintained without serious adverse reactions throughout 6 month follow-up period. Microbiological evaluations of donor tissue at the time of thawing and tissue preparation were done, and the results were all negative. Tissue that was taken intraoperatively from the recipient cornea also showed negative microbiological results. In conclusion, tectonic lamellar keratoplasty, using cryopreserved corneal tissue, only onto the remaining Descemet's membrane and endothelium in an emergent condition, was a safe and effective treatment.
Ophthalmic emergencies such as corneal perforation or impending corneal perforation require immediate detection and prompt intervention. The integrity of the cornea should be restored as soon as possible using tissue adhesives, conjunctival flaps, amniotic membrane transplantation, or penetrating keratoplasty (PKP).1
Of those, PKP provides a good corneal tissue substitute. However, in cases of PKP with an uncontrolled infection, the remaining diseased corneal tissue may persistently harbor pathogens, and removal of the recipient's endothelium may increase the risk of causative organisms spreading into the anterior chamber and causing endophthalmitis. In addition, a graft-versus-host reaction between the donor tissue and the recipient may result in graft failure and visual loss.23 Therefore, it is important to select the right surgical method and corneal substitute to obtain a better visual outcome.
Cryopreservation provides preserved stability of the tissues, and therefore, can be used for preserving tissues for urgent clinical use. However, a critical drawback of cryopreserved corneal use is impaired endothelial cell function. To allow the application of cryopreserved cornea, surgical methods that conserve the endothelium of the recipient are needed.
In the following report, we describe the treatment course and surgical outcomes of tectonic lamellar keratoplasty, using cryopreserved cornea to rescue an abrupt stromal melting with a large descemetocele.
A 76-year-old male patient presented with 4 days of persistent ocular pain. After traumatic cataract extraction 30 years prior, he underwent PKP 3 years ago due to pseudophakic bullous keratopathy. The patient was taking 200 mg of oral acyclovir, topical levofloxacin, and topical prednisolone acetate for frequently recurring herpetic keratitis. Examination revealed that his cornea was edematous and entirely opaque with 7×7 mm sized epithelial defects. His visual acuity was light perception, and ultrasonography confirmed no definite change of vitreous and retina. The patient was given topical moxifloxacin, fortified with cefazolin and tobramycin, every 2 hours, and additionally, he was given an oral acyclovir dosage of 600 mg, and a topical acyclovir ointment. Two days after changing medications, the patient visited the clinic and showed improved vision, but microscopic examination revealed only a very thin layer of stroma and Descemet's membrane, with the endothelium remaining (Fig. 1A).
Donor cornea was prepared from the whole globe, without any contraindicative features at the Yonsei University Eye Bank, cryopreserved at -80℃ for 3 months. The whole globe was sequentially thawed at 20℃ for 4 hours, at 4℃ for 4 hours, and at room temperature for 2 hours to minimize thawing-related tissue stress. After the whole globe was completely thawed, a corneal button was removed, and then preserved in Optisol GS® (Bausch & Lomb, Irvine, CA, USA) for 2 hours until surgery.
Tectonic lamellar keratoplasty was done with prepared cornea. Completely thawed donor tissue was trephined by the size of 8.25 mm. The donor cornea, with intact entire layers, was placed on top of the recipient's descemetocele and was sutured to the host's eye bed, using interrupted 10-0 nylon, to avoid penetrating the descemetocele. Topical moxifloxacin and 2% voriconazole were initiated every 2 hours, immediately after the operation. At postoperative 2 months, topical moxifloxacin was discontinued, and levofloxacin and rimexolone were initiated. Oral anti-inflammatory medications, 12 mg of triamcinolone and 300 mg of cyclosporine, were given from 1 week after surgery.
Donor-recipient interfaces were evaluated 10 days, 1 month, and 6 months after the operation, using corneal optical coherence tomography (OCT) (RTVue; Optoview, Inc., Fremont, CA, USA).
Microbiological evaluations were done on donor tissues that were biopsied during and after thawing, and on the recipient's tissue that was cultured before grafting. Bacterial, fungal and viral assessments all showed negative results, even though the assessment for viral infection indicated that the amount of sample was insufficient for viral polymerase chain reaction. Because cryopreserved donor corneas are devoid of immune cells,4 we delayed immune modulating agents for the first week to reduce the chance of recurrent infection. However, the graft survived without complications (Fig. 1B). Serially anterior segmented OCT images revealed well attached double layers of Descemet's membrane and endothelium between the graft and the host (Fig. 2). The interface showed mild opacity which could slightly interfere with visual function. At 6 months postoperatively, the uncorrected and corrected visual acuity was 20/400, the keratometry value was 46.25/50.25 with axis of 163, and intraocular pressure was 11 mm Hg.
Infectious corneal ulcer with significant melting of the corneal stromal tissue must be immediately corrected with a corneal substitute. Especially, corneal ulcer with infiltration that progresses deeper into the stroma should be treated with prompt intervention to restore ocular structural integrity. However, fresh corneal tissue is not always available in emergency situations, and substitutes for corneal tissue are needed in those cases. Based on previous studies, cryopreserved cornea provided a source of tissue for deep anterior lamellar keratoplasty (DALK) which was timely and safe.5
In our present case, the recipient's Descemet's membrane and endothelium were preserved. Because the causative organisms which induced corneal stromal melting were not identified, the remaining recipient tissue was preserved to avoid spread into the anterior chamber. Removal of Descemet's membrane and endothelium could induce additional tissue damage which could cause more tissue reaction at the interface. As the surgical outcome demonstrated, the donor's frozen-thawed Descemet's membrane and the host corneal bed endothelium caused only a mild opacity at the interface of the graft-host junction.
Rejection did not occur in our patient. However, using the same approach, Javadi, et al.6 noted 1 case of subepithelial rejection in 15 eyes that underwent PKP, using cryopreserved graft. In contrast, Chen, et al.5 and Li, et al.7 observed no episodes of graft after DALK, when using glycerol cryopreserved corneal tissue grafts. Therefore, different cryopreservation media may differently alter corneal antigenicity, and further studies are needed to identify the most appropriate preservation media.
For the emergent lamellar keratoplasty, the use of cryopreserved donor cornea effectively maintained ocular integrity, improved vision, provided infection control, and showed no graft rejection for 6 months of follow-up.
Figures and Tables
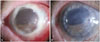 | Fig. 1Slit lamp photograph of cornea taken preoperatively and postoperatively. (A) A photograph of the cornea 1 day before the operation. The stroma of the corneal graft melted down with preservation of only the Descemet's membrane and endothelium. (B) Six months after the operation, an intact corneal graft with mild interface opacity is shown. |
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2044).
References
1. Krachmer JH, Mannis MJ, Holland EJ. Cornea. 2nd ed. Philadelphia: Elsevier Mosby;2005.
2. Watson SL, Tuft SJ, Dart JK. Patterns of rejection after deep lamellar keratoplasty. Ophthalmology. 2006; 113:556–560.

3. Al-Torbak A, Malak M, Teichmann KD, Wagoner MD. Presumed stromal graft rejection after deep anterior lamellar keratoplasty. Cornea. 2005; 24:241–243.

4. Yao YF, Zhang YM, Zhou P, Zhang B, Qiu WY, Tseng SC. Therapeutic penetrating keratoplasty in severe fungal keratitis using cryopreserved donor corneas. Br J Ophthalmol. 2003; 87:543–547.

5. Chen W, Lin Y, Zhang X, Wang L, Liu M, Liu J, et al. Comparison of fresh corneal tissue versus glycerin-cryopreserved corneal tissue in deep anterior lamellar keratoplasty. Invest Ophthalmol Vis Sci. 2010; 51:775–781.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download