Abstract
Among various surgical methods introduced to optimize esthetic results, robotic surgery has gradually expanded in scope. As incision, approach, and operation view in robotic surgery differ from existing surgical methods, we should consider reconstruction from a different perspective. We recently experienced two mandibular reconstruction cases after tumor ablative surgery with robotic neck dissection using the conventional reconstruction method and virtual surgical planning (VSP), respectively. We found that the conventional reconstruction method is inappropriate in modified facelift incision in robotic neck dissection because it provides limited surgical scope, restricts access to the defect area, and therefore, consumes considerable time before anastomosis. For these reasons, the authors consider VSP far more viable in the era of robotic surgery.
With developments in computer technology and increased interest in esthetics, virtual surgical planning (VSP) and robotic surgery have recently come into wide use. Because VSP allows the realization of unique shapes and contours, we expect its application in mandibular reconstruction to increase. Although critics point to the time and expense associated with VSP in terms of pre-operative simulation and prototyping, these are more than compensated for by the convenience during surgery, particularly given the rising trend toward robotic surgery. We recently experienced two cases of posterior segmental mandibulectomy and reconstruction in oral cavity cancer via the intraoral and modified facelift approach. One surgery was performed with VSP and the other was not. This report presents, based on our experience, the shortcomings of surgery without VSP when combined with robot surgery using modified face-lift incision, and we emphasize the necessity for VSP in the era of robotic surgery.
Two female patients were referred from the Department of Otorhinolaryngology for segmental mandibulectomy and mandible reconstruction. Each patient was diagnosed as chondrosarcoma on the right mandible and oral squamous cell carcinoma on left lower gingiva with bone invasion, respectively. VSP was applied in the first case, with mandible resection guide, fibula cutting guide, pre-bend reconstruction plate, and a rapid prototyping (RP) model being prepared before surgery (Fig. 1). In the second case, however, it was decided to treat the patient without computer simulation, and no additional appliances were prepared.
VSP for the first case was made using 3-dimensional (3D) simulation software (Mimics 16.0, Materialize, Leuven, Belgium). We first imported the Digital Imaging and Communications in Medicine (DICOM) files of facial bone and fibula into the software. 3D objects of mandible and fibula were calculated and obtained. Next, virtual segmental mandibulectomy was done considering the safety margin. Fibula was osteotomized with the same plane used in mandibulectomy. After removing the segmented mandible, we put the osteotomized fibula into the defect. The reconstructed mandible was converted into stereolithography (STL) files and sent to a company (Med CEP TECH Co., Seoul, Korea) for RP model and guides.
The surgical procedure, overall similar in both patients, was as follows: first, neck dissection was done by an otorhinolaryngologist who was highly skillful in robotic surgery. The mandibular lesion was exposed through intraoral incision. A tunnel was made between the modified face-lift and intraoral incision, and a reconstruction plate was applied through it. The reconstruction plate was fixed to maintain condyle position after segmental mandibulectomy and removed immediately. Following the segmental madibulectomy, the reconstruction plate was reapplied. The fibula flap was then positioned on the defect and fixed with miniplate and screws through the intraoral (anterior) and modified facelift (posterior) incisions. Finally, microvascular surgery was performed as usual.
The pathological surgical margin was reported as "free of tumor" in both cases. The patient using VSP experienced a successful result with symmetrically reconstructed mandible (Fig. 2A). We can identify survived flap and bone remodeling in 5 month follow-up panoramic view (Fig. 2B). Unfortunately, however, the flap on the patient without VSP failed (Fig. 2C).
Appearance is an important parameter affecting quality of life (QOL) in patients with malignant tumor, having been ranked 3rd in QOL affecting parameters in patients with oral squamous cell carcinoma.1 Recently, there have been many trials to optimize esthetic results in various types of tumor surgery and in reconstruction of mandible.2345678 Among them, VSP and robotic surgery are coming into general use. Together, they enable a cost-effective procedure and shorten the operation time compared with traditional planning. We can also establish and apply an ideal treatment plan to the patient using VSP.910 VSP in particular is emerging as essential for mandibular reconstruction because of the limited surgical field in the modified face-lift incision, used for robotic neck dissection for oral cavity cancer.
First of all, VSP delimits the surgical field by identifying the margin. García-Díez, et al.3 reported excellent access to the posterior mandible and a limited surgical field, using the rhytidectomy approach for mandibular reconstruction, in terms of microvascular surgery. We agree with some of these observations in regard to microvascular surgery. However, our experience indicates somewhat otherwise. We could not identify the anterior and posterior margins in one view, as is possible using conventional incision methods such as the apron flap incision. Also, we could not precisely identify the posterior resection margin through the modified face-lift incision, particularly when the margin is close to condyle. VSP reduces the need for frequent margin determination through the intraoral and modified facelift incisions when a resection guide is applied.
Second, it is difficult to access the defect area through the unprocessed flap itself, especially with a longer flap. Unlike in the case of a conventional neck incision, we must repeatedly adjust the length and contour through a tunnel incision in robotic surgery. This not only causes unnecessary and unpredictable trauma to the flap, but consumes time. Using VSP, we can easily obtain proper flap length and contour with a cutting guide.7 Although we can approximate the flap contour with a prefabricated framework under the RP model, contour and length accuracy are both sacrificed. A better outcome thus calls for the RP model as well as VSP.
Third, surgery without VSP may require extended ischemic time, which has an adverse effect on flap survival. This relates to the shape of the flap in robotic surgery. Nothing takes longer than adjusting the flap through a tunnel incision to achieve proper length and contour. Several factors affect flap survival (Table 1), and some of them are still controversial. According to Wong, et al.,11 and Pattani, et al.,12 prolonged operative time significantly affects microvascular flap failure. Chang, et al.13 also reported longer ischemic time as a significant risk factor for flap loss. Although prolonged operative and ischemic time are not the only factors affecting flap failure, they might have an adverse effect on flap survival. In our two cases, total operation time was thirteen and eighteen hours, and the second case took twice long from harvesting of the flap to beginning microvascular anastomosis, 6 rather than 3 hours.
Given the complex effects of the problems mentioned above, more time and effort are required for non-VSP surgery, especially in robotic surgery using modified facelift incision. In the future, robotic surgery with modified facelift incision will be universalized to achieve better esthetic outcomes. The use of VSP should be expanded along as surgical techniques continue to develop. Although somewhat limited, our experience points to a greater role for VSP in the era of robotic surgery.
Figures and Tables
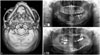 | Fig. 2Post-operative imaging study. (A) Immediate postoperative 3-dimensional image operated with virtual surgical planning showing symmetrically reconstructed mandible. (B) Five-month follow-up panoramic view operated with virtual surgical planning showing well remodeled grafted bone. (C) Seven-month follow-up panoramic view operated without virtual surgical planning showing necrosis of grafted bone. |
ACKNOWLEDGEMENTS
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094027).
References
1. Handschel J, Naujoks C, Hofer M, Krüskemper G. Psychological aspects affect quality of life in patients with oral squamous cell carcinomas. Psychooncology. 2013; 22:677–682.

2. Bianchi B, Ferri A, Ferrari S, Copelli C, Sesenna E. Facelift approach for mandibular resection and reconstruction. Head Neck. 2014; 36:1497–1502.

3. García-Díez EM, Cho-Lee GY, Raigosa-García JM, Sieira-Gil R, Martí Pagès C. Rhytidectomy approach for mandibular reconstruction with microvascular free flaps after resection of mandibular benign tumors. J Oral Maxillofac Surg. 2013; 71:2156–2168.

4. Koh YW, Chung WY, Hong HJ, Lee SY, Kim WS, Lee HS, et al. Robot-assisted selective neck dissection via modified face-lift approach for early oral tongue cancer: a video demonstration. Ann Surg Oncol. 2012; 19:1334–1335.

5. Nkenke E, Agaimy A, von Wilmowsky C, Eitner S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma. J Oral Maxillofac Surg. 2013; 71:1983–1992.

6. Yang X, Hu J, Zhu S, Liang X, Li J, Luo E. Computer-assisted surgical planning and simulation for condylar reconstruction in patients with osteochondroma. Br J Oral Maxillofac Surg. 2011; 49:203–208.

7. Zheng GS, Su YX, Liao GQ, Chen ZF, Wang L, Jiao PF, et al. Mandible reconstruction assisted by preoperative virtual surgical simulation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:604–611.

8. Kim JY, Cho H, Cha IH, Nam W. Esthetic neck dissection using an endoscope via retroauricular incision: a report of two cases. J Korean Assoc Oral Maxillofac Surg. 2014; 40:27–31.

9. Xia JJ, Phillips CV, Gateno J, Teichgraeber JF, Christensen AM, Gliddon MJ, et al. Cost-effectiveness analysis for computer-aided surgical simulation in complex cranio-maxillofacial surgery. J Oral Maxillofac Surg. 2006; 64:1780–1784.

10. Zhao L, Patel PK, Cohen M. Application of virtual surgical planning with computer assisted design and manufacturing technology to cranio-maxillofacial surgery. Arch Plast Surg. 2012; 39:309–316.

11. Wong AK, Joanna Nguyen T, Peric M, Shahabi A, Vidar EN, Hwang BH, et al. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery. 2015; 35:6–12.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download