Abstract
The epithelial cytokine response, associated with reactive oxygen species (ROS), is important in Helicobacter pylori (H. pylori)-induced inflammation. H. pylori induces the production of ROS, which may be involved in the activation of mitogen-activated protein kinases (MAPK), janus kinase/signal transducers and activators of transcription (Jak/Stat), and oxidant-sensitive transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and thus, expression of interleukin-8 (IL-8) in gastric epithelial cells. α-lipoic acid, a naturally occurring thiol compound, is a potential antioxidant. It shows beneficial effects in treatment of oxidant-associated diseases including diabetes. The present study is purposed to investigate whether α-lipoic acid inhibits expression of inflammatory cytokine IL-8 by suppressing activation of MAPK, Jak/Stat, and NF-κB in H. pylori-infected gastric epithelial cells. Gastric epithelial AGS cells were pretreated with or without α-lipoic acid for 2 h and infected with H. pylori in a Korean isolate (HP99) at a ratio of 300:1. IL-8 mRNA expression was analyzed by RT-PCR analysis. IL-8 levels in the medium were determined by enzyme-linked immunosorbent assay. NF-κB-DNA binding activity was determined by electrophoretic mobility shift assay. Phospho-specific and total forms of MAPK and Jak/Stat were assessed by Western blot analysis. ROS levels were determined using dichlorofluorescein fluorescence. As a result, H. pylori induced increases in ROS levels, mRNA, and protein levels of IL-8, as well as the activation of MAPK [extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun NH2-terminal kinase 1/2 (JNK1/2), p38], Jak/Stat (Jak1/2, Stat3), and NF-κB in AGS cells, which was inhibited by α-lipoic acid. In conclusion, α-lipoic acid may be beneficial for prevention and/or treatment of H. pylori infection-associated gastric inflammation.
Helicobacter pylori (H. pylori) infection mediates gastritis and gastric adenocarcinoma.1 Interleukin-8 (IL-8) contributes to gastric inflammation.2 IL-8 levels are found to be elevated in gastric mucosal tissues of the patients infected with Helicobacter pylori2 and H. pylori-infected gastric epithelial cells.3,4 Reactive oxygen species (ROS) mediate the expression of IL-8 by activating oxidant-sensitive transcription factors, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator protein-1 (AP-1), janus kinase/signal transducers, and activators of transcription (Jak/Stat).345
H. pylori exhibits chemotactic activity by inducing neutrophil activation, and these activated neutrophils induce ROS production.67 It was recently reported that ROS is involved in Jak/Stat signal molecules in inflammatory signaling pathway of non-phagocytic cells, as well as phagocytic cells. Jak/Stat signaling mediates activation of cytokine signaling.89
There are three subfamilies of mitogen-activated protein kinases (MAPKs); extracellular signal-regulated kinases (ERKs), c-Jun NH2-terminal protein kinases (JNKs), and p38 MAPK. The cytotoxin-associated gene (cagA) pathogenicity island of H. pylori is involved in NF-κB and MAPK activation in gastric epithelial cells.3 Transcription of IL-8 gene requires NF-κB activation and NF-κB is indispensable for the enhanced IL-8 mRNA transcription in H. pylori-infected gastric epithelial cells.347
α-lipoic acid (α-LA) is supplied from diets such as spinach and broccoli and from a supplement. α-LA and its active reduced counterpart dihydrolipoic acid (DHLA) reduce oxidative stress by chelating transition metals, recycling endogenous antioxidants, and scavenging ROS.1011 α-LA showed beneficial effect on treating ROS-mediated diseases including diabetes, atherosclerosis, and hypertension.12131415
Therefore, we investigated whether α-LA reduces levels of ROS produced in H. pylori-infected gastric epithelial cells, thereby suppressing the activation of inflammatory signaling molecules, such as MAPK (ERK1/2, JNK1/2, p38), Jak/Stat (Jak1, Jak2, Stat3), transcription factor NF-κB, and IL-8 expression in H. pylori-infected gastric epithelial cells.
A human gastric epithelial cell line AGS (gastric adenocarcinoma, ATCC CRL 1739) was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured as previously described.3 H. pylori strain in a Korean isolate (HP99; cagA+, vacA s1b, m2, iceA genotype) was inoculated onto chocolate agar plates at 37℃ under microaerophilic conditions using GasPak™ EZ Gas Generating Pouch Systems (BD Biosciences, San Jose, CA, USA).3 Prior to infection, H. pylori were harvested, and then resuspended in antibiotic-free cell culture medium. H. pylori was added to cultured cells at a bacterium/cell ratio 300:1.
For time-course experiment for IL-8 levels, cells were infected with H. pylori for several time points. α-LA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in ethanol. The cells were pretreated with α-LA (final concentrations of 10 and 20 µM) for 2 h and then infected with H. pylori for 30 min (for ROS levels, NF-κB, p-IκBα, IkBα, MAPK, Jak/Stat), 3 h (for IL-8 mRNA) or 12 h (for IL-8 protein levels). None and control cells without α-LA received ethanol instead of α-LA. The time points for determining ROS, NF-κB, p-IκBα, IκBα, MAPK, and Jak/Stat, as well as 2 h-pretreatment of α-LA, were adapted from our previous studies.161718
IL-8 levels in the medium were determined by using enzyme linked immunosorbent assay (ELISA) kits (Biosource International, Inc., San Diego, CA, USA) following the manufacturer's instructions. For real-time PCR analysis, total RNA in cells were isolated and converted into cDNA by reverse transcription process using a random hexamer and virus reverse transcriptase (Promega, Madison, WI, USA). Sequences of IL-8 primers and β-actin were adapted from our previous study.19 cDNA was added in a SYBR Green Realtime PCR Master Mix (TOYOBO Co., Osaka, Japan) containing 10 pg/mL of forward and reverse primers for IL-8. cDNA was amplified by 40 cycles, denaturation at 95℃ for 15 sec, annealing at 60℃ for 5 sec, and extension at 72℃ for 30 sec. β-actin gene was amplified in the same reaction to serve as the reference gene.
ROS levels were determined using 2',7'-dichlorodihydrofluorescein diacetate (Invitrogen, Carlsbad, CA, USA) as previously described.20 The amount of ROS trapped in the cells was expressed as the relative increase over the ROS level in cells cultured in the absence of H. pylori, which was set at 100.
NF-κB-DNA binding activity was determined by electrophoretic mobility shift assay (EMSA) as previously described.4
For Western blot analysis, proteins in whole cell extracts were subjected to 6% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes which was blocked using 3-5% nonfat dry milk in Tris-buffered saline and 0.2% Tween 20 (TBS-T) for 2 h at room temperature. The membranes were incubated with antibodies for total and phospho-specific forms of ERK1/2, JNK1/2, p38, Stat3, Jak1, and Jak2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted in TBS-T containing 3% dry milk at 4℃ for 16 h. After washing with TBS-T, primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (anti-goat or anti-rabbit), respectively, and visualized by the enhanced chemiluminescence (ECL) detection system (Santa Cruz Biotechnology) according to the manufacturer's instruction.
The statistical differences were determined using one-way ANOVA and Newman-Keul's test. All values are expressed as mean±standard error of means (SEM) of four different experiments. A value of p<0.05 was considered statistically significant.
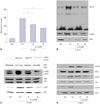
As shown in Fig. 1A and B, H. pylori infection induced mRNA expression of IL-8 time-dependently. IL-8 levels in the medium were also increased by H. pylori infection. H. pylori-induced increases in IL-8 mRNA levels were maximized at 3 h, while IL-8 protein levels in the medium stably increased from 8 h-culture. Therefore, to examine the effect of α-LA on H. pylori-induced expression of IL-8, AGS cells were pretreated with α-LA and cultured in the presence of H. pylori for 3 h (to assess mRNA levels) and 8 h (to assess protein levels in the medium) (Fig. 1C and D). α-LA showed inhibitory effect on H. pylori-induced IL-8 expression at both mRNA and protein levels in a dose-dependent manner.
To investigate whether α-LA inhibits H. pylori-induced increases in ROS levels and activation of NF-κB, intracellular ROS levels, NF-κB DNA binding activity, and levels of total and phospho-specific forms of IκBα were determined in the cells infected with H. pylori treated with or without α-LA. α-LA suppressed H. pylori-induced increases in ROS levels and NF-κB activation, accompanied with inhibition of phosphorylation of IκBα and a decrease in the total forms of IκBα (Fig. 2A and B). As shown in Fig. 2C, α-LA suppressed H. pylori-induced phosphorylation of ERK1/2, JNK1/2, and p38, while total forms of ERK1/2, JNK1/2, and p38 were not affected by H. pylori infection or α-LA treatment.
We investigated whether Jak-Stat signaling is involved in H. pylori-induced inflammation. As shown in Fig. 2D, H. pylori infection induced phosphorylation of Stat3, accompanied with phosphorylation of Jak1 and Jak2 in AGS cells, which was inhibited by α-LA dose-dependently. Total forms of Stat3, Jak1, and Jak2 were not changed by H. pylori infection or α-LA treatment. The results suggest that MAPK and Jak1/2-Stat3 pathways mediate H. pylori-induced IL-8 expression, which is inhibited by α-LA.
In the present study, we found that H. pylori, Korean isolate (HP99), induces IL-8 expression and activation of MAPK, Jak/Stat, and NF-κB, which were inhibited by α-LA. Since ROS mediates activation of MAPK, Jak/Stat, and NF-κB cells,1718 the inhibitory effect of α-LA on ROS production may suppress H. pylori-induced signaling for IL-8 expression in AGS cells. Several studies have reported that MAPK inhibitors, U0126 (an ERK inhibitor), and SB203580 (a p38 inhibitor) suppressed NF-κB activation in H. pylori-infected AGS cells.3 These results indicate that NF-κB activation acts as a downstream of ERK and/or p38 signaling in H. pylori-infected AGS cells. Therefore, α-LA may inhibit H. pylori-induced IL-8 expression through suppression of MAPK-mediated NF-κB activation in AGS cells.
Additionally, we found that α-LA inhibits H. pylori-induced Stat3 activation in AGS cells. Jak-Stat signaling is responsible for various cellular responses to cytokines, growth factors, and hormones.21 Bronte-Tinkew, et al.22 demonstrated that H. pylori activates Stat3 in gastric epithelial cells. Inhibition of Jak/Stat activation with chemical inhibitors suppresses phosphorylation of ERK, indicating that ERK/NF-κB signaling acts as a downstream of Jak2 activation.23 For phosphorylation of Stat3, activation of Jak is required. Therefore, Jak may be phosphorylated prior to activation of Stat3 by infection of H. pylori. In the present study, both Jak1 and 2 were phosphorylated along with phosphorylation of Stat3 in AGS cells. Since H. pylori induces actvation of MAPK and Jak/Stat at 30 min and NF-κB activation at 1 h-culture, Jak/Stat and MAPK may be upstream signaling of NF-κB in HP99-infected AGS cells. Since α-LA reduces ROS levels and activation of MAPK, Jak1/2-Stat3, and NF-κB, and thus, IL-8 expression, α-LA may have a therapeutic potential for H. pylori infection-associated inflammation.
Figures and Tables
Fig. 1
mRNA and protein levels of IL-8 in H. pylori-infected AGS cells treated with or without α-LA. (A and B) The cells were cultured in the presence of H. pylori for indicated time points. mRNA levels of IL-8 were determined by real-time PCR. IL-8 mRNA levels were normalized to β-actin (A). IL-8 levels in the medium were assessed by ELISA (B). (C and D) The cells were pre-treated with α-LA for 2 h, and cultured in the presence of H. pylori for 3 h (IL-8 mRNA level, C) or 8 h (IL-8 level in the medium, D). All values are expressed as mean±SEM of four different experiments. *p<0.05 vs. 0 h (A and B) or control (C and D). Non (none), the cells cultured in the absence of H. pylori without treatment of α-LA; Con (control), the cells cultured in the presence of H. pylori without treatment of α-LA. H. pylori, Helicobacter pylori; α-LA, α-lipoic acid; ELISA, enzyme linked immunosorbent assay; IL, interleukin; SEM, standard error of means; AGS, gastric adenocarcinoma.

Fig. 2
ROS levels, activation of NF-κB, MAPK, and Jak/Stat in H. pylori-infected AGS cells treated with or without α-LA. The cells were pretreated with α-LA for 2 h and cultured in the presence of H. pylori for 30 min (ROS levels, activation of NF-κB, MAPK, and Jak/Stat) or 1 h (NF-κB). (A) ROS levels were determined using DCF fluorescence. All values are expressed as mean±SEM of four different experiments. (B) NF-κB activation was determined using EMSA, performing western blotting for phospho- and total forms of IκBα. (C and D) The levels of phospho-specific and total forms of MAPK (ERK1/2, JNK1/2, p38, C) and Jak1, Jak2, Stat3 (D) in whole cell lysates were determined by Western blot analysis. Non (none), the cells cultured in the absence of H. pylori without treatment of α-LA; Con (control), the cells cultured in the presence of H. pylori without treatment of α-LA. *p<0.05 vs. control. H. pylori, Helicobacter pylori; ROS, reactive oxygen species; MAPK, mitogen-activated protein kinases; α-LA, α-lipoic acid; EMSA, electrophoretic mobility shift assay; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal protein kinase; Jak/Stat, janus kinase/signal transducers and activators of transcription; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AGS, gastric adenocarcinoma; DCF, 2',7'-dichlorodihydrofluorescein.
ACKNOWLEDGEMENTS
This study was supported by a grant from the NRF of Korea, funded by the Korean government (NRF-2012R1A1A2043423) and by the program of local joint research of the graduate student, Dispatch of Academic Subsequent Generation as a part of the International Joint Research Program in 2008. Authors wish to express appreciation to Dr. T. Morio (Tokyo Medical and Dental University) for valuable discussion and technical support for initial work of JH Choi.
References
1. Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002; 2:28–37.

2. Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995; 48:133–136.

3. Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004; 84:49–62.

4. Kim H. Oxidative stress in Helicobacter pylori-induced gastric cell injury. Inflammopharmacology. 2005; 13:63–74.

5. O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002; 109:S121–S131.
6. Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001; 36:706–716.

7. Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003; 48:257–265.
8. Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007; 282:31460–31468.

9. Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, et al. Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006; 9:101–110.

10. Fujita H, Shiosaka M, Ogino T, Okimura Y, Utsumi T, Sato EF, et al. Alpha-lipoic acid suppresses 6-hydroxydopamine-induced ROS generation and apoptosis through the stimulation of glutathione synthesis but not by the expression of heme oxygenase-1. Brain Res. 2008; 1206:1–12.

11. Kishi Y, Schmelzer JD, Yao JK, Zollman PJ, Nickander KK, Tritschler HJ, et al. Alpha-lipoic acid: effect on glucose uptake, sorbitol pathway, and energy metabolism in experimental diabetic neuropathy. Diabetes. 1999; 48:2045–2051.

12. Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004; 11:1135–1146.

13. Aly HA, Lightfoot DA, El-Shemy HA. Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem Biol Interact. 2009; 182:112–118.

14. Zhang H, Jia H, Liu J, Ao N, Yan B, Shen W, et al. Combined R-alpha-lipoic acid and acetyl-L-carnitine exerts efficient preventative effects in a cellular model of Parkinson's disease. J Cell Mol Med. 2010; 14:215–225.

15. Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002; 33:83–93.

16. Byun E, Lim JW, Kim JM, Kim H. α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells. Mediators Inflamm. 2014; 2014:380830.
17. Cho SO, Lim JW, Kim KH, Kim H. Diphenyleneiodonium inhibits the activation of mitogen-activated protein kinases and the expression of monocyte chemoattractant protein-1 in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm Res. 2011; 60:501–507.

18. Cha B, Lim JW, Kim KH, Kim H. 15-deoxy-D12,14-prostaglandin J2 suppresses RANTES expression by inhibiting NADPH oxidase activation in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol. 2011; 62:167–174.
19. Lee SE, Lim JW, Kim JM, Kim H. Anti-inflammatory mechanism of polyunsaturated fatty acids in Helicobacter pylori-infected gastric epithelial cells. Mediators Inflamm. 2014; 2014:128919.
20. Ito A, Uehara T, Tokumitsu A, Okuma Y, Nomura Y. Possible involvement of cytochrome c release and sequential activation of caspases in ceramide-induced apoptosis in SK-N-MC cells. Biochim Biophys Acta. 1999; 1452:263–274.

21. Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002; 285:1–24.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download