Abstract
Purpose
Apoptosis of vascular endothelial cells is a type of endothelial damage that is associated with the pathogenesis of cardiovascular diseases such as atherosclerosis. Heterotrimeric GTP-binding proteins (G proteins), including the alpha 12 subunit of G protein (Gα12), have been found to modulate cellular proliferation, differentiation, and apoptosis of numerous cell types. However, the role of Gα12 in the regulation of apoptosis of vascular cells has not been elucidated. We investigated the role of Gα12 in serum withdrawal-induced apoptosis of human umbilical vein endothelial cells (HUVECs) and its underlying mechanisms.
Materials and Methods
HUVECs were transfected with Gα12 small-interfering RNA (siRNA) to knockdown the endogenous Gα12 expression and were serum-deprived for 6 h to induce apoptosis. The apoptosis of HUVECs were assessed by Western blotting and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. The expressions of microRNAs were analyzed by quantitative real-time PCR.
Results
Knockdown of Gα12 with siRNA augmented the serum withdrawal-induced apoptosis of HUVECs and markedly repressed the expression of microRNA-155 (miR-155). Serum withdrawal-induced apoptosis of HUVECs was inhibited by the overexpression of miR-155 and increased significantly due to the inhibition of miR-155. Notably, the elevation of miR-155 expression prevented increased apoptosis of Gα12-deficient HUVECs.
G proteins play important roles in cells as signal transducers that convert extracellular signals into intracellular signals. G proteins comprise alpha, beta, and gamma subunits (Gα, Gβ, and Gγ, respectively), which couple to seven-transmembrane domain receptors called G protein-coupled receptors (GPCRs). Activation of GPCRs induces the exchange of GDP with GTP in the Gα subunit, which leads to the activation of G proteins and dissociation of the Gα subunit from the Gβγ subunit.1 Gα proteins are grouped into four families: Gs, Gi, Gq, and G12. Among the four families, Gα12 was discovered last, and its roles in the modulation of cellular responses including proliferation, differentiation, and apoptosis have not been elucidated fully. Gα12 protein has been found to act as a potent oncogene and to stimulate the invasiveness of human cancer cells.23
MicroRNAs (miRNAs), small noncoding RNAs, have been found to regulate the gene expression by posttranscriptional regulation, which results in the silencing of target genes. As miRNAs are involved in the regulation of many cellular functions, misregulated expression of miRNAs can lead to many diseases, including cancer and atherosclerosis.45 MicroRNA-155 (miR-155) is encoded from the processing of the B cell integration cluster gene and is known to be involved in oncogenesis and immune responses. Recently, miR-155 has been also found to play a crucial role in the pathogenesis of atherosclerosis, including the migration of vascular smooth muscle cells (VSMCs), lipid uptake by macrophages, and inflammation in and apoptosis of endothelial cells.6 However, studies of the effects of miR-155 on atherosclerosis have produced conflicting results, and further study is needed to elucidate the role of miR-155 in atherosclerosis.
Many studies have shown that the G protein-signaling system can modulate the apoptosis of numerous cells;7 however, it is not known whether Gα12 signaling can regulate the apoptosis of vascular cells. Thus, we investigated whether Gα12 affects apoptosis induced by serum deprivation in human umbilical vein endothelial cells (HUVECs). We found that Gα12 protected HUVECs from serum withdrawal-induced apoptosis through the regulation of miR-155 expression.
HUVECs were purchased from Invitrogen (Carlsbad, CA, USA) and were grown in endothelial growth medium-2 (EGM-2) supplemented with 2% fetal bovine serum and an EGM-2 Bullet Kit (Lonza, Walkersville, MD, USA). Cells were cultured in 5% CO2 at 37℃ in an incubator and were used between passages 4 and 7 in this study.
Synthetic small-interfering RNA (siRNA), miR-155 mimic, or miR-155 inhibitor were transfected into cells using Lipofectamine 2000 reagents according to the manufacturer's guidelines (Invitrogen, Carlsbad, CA, USA). The following materials were used: siRNA targeting Gα12 (Thermo Scientific Dharmacon, Lafayette, CO, USA); control siRNA (Stealth siRNA duplex, Invitrogen, Carlsbad, CA, USA); miR-155 mimics (sense: 5'-CUCCUACAUAUUAGCAUUAACA-3'; antisense: 5'-UGUUAAUGCUAAUAUGUAGGAG-3', Genolution, Seoul, Korea); miRNA for negative control (sense: 5'-CCUCGUGCCGUUCCAUCAGGUAG-3'; antisense: 5'-CUACCUGAUGGAACGGACGAGG-3'); and miR-155 inhibitor, which comprised single-stranded RNA oligonucleotides conjugated with 2'-O-methoxyethyl phosphorothioate (Genolution, Seoul, Korea).
Immunoblot analysis was performed as described previously.8 Cell lysates were subjected to SDS-PAGE, and the resultant blot was analyzed with specific antibodies: antibodies against Gα12 and β-actin, which were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and antibodies against cleaved caspase-3 and phosphorylated p38 mitogen-activated protein kinase (MAPK) (Thr180/Tyr182), purchased from Cell Signaling Technology (Beverly, MA, USA).
The expression of miRNA was analyzed by quantitative real-time PCR (RT-PCR) as described previously.9 In HUVECs, miRNAs were isolated using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA) according to the manufacturer's instructions and were reverse transcribed with a TaqMan MicroRNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) using miRNA sequence-specific primers for miR-17-3, miR-31, miR-155, and miR-191. Briefly, for the RT reactions, 30 ng of miRNA was used in each reaction (15 µL) and was mixed with the RT primer (3 µL). The RT reaction was performed at 16℃ for 30 min, 42℃ for 30 min, and 85℃ for 5 min. MiRNA levels were quantified via RT-PCR (StepOnePlus; Applied Biosystems) using TaqMan MicroRNA assays. The amplification steps included denaturation at 95℃, followed by 40 cycles of denaturation at 95℃ for 15 s and then annealing at 60℃ for 1 min. All reactions, including controls, were performed in triplicate. Relative expression of miRNAs was analyzed using the 2-ΔΔCt method and was normalized via RNU6B expression for all samples.
HUVECs were seeded in the confocal dish, deprived of serum to induce apoptosis, and then fixed in 4% paraformaldehyde for the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay according to the manufacturer's instructions (Roche, Nutley, NJ, USA). Cells were counterstained with propidium iodide to visualize all of the nuclei. Stained cells were viewed under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
To examine the effects of serum withdrawal on HUVEC apoptosis, HUVECs were deprived of serum for the indicated times, and apoptosis was assessed via Western blotting, using an antibody to cleaved caspase-3 and a TUNEL assay to label apoptotic cells, which were then viewed by fluorescent microscopy. Serum withdrawal induced apoptosis in a time-dependent manner. Apoptosis of HUVECs appeared within 3 h of serum starvation and continued until 24 h after serum starvation (Fig. 1A and B). To investigate the role of Gα12 in serum withdrawal-induced apoptosis, HUVECs were transfected with Gα12 siRNA to knock down the expression of endogenous Gα12 and were then serum deprived for 6 h to induce apoptosis. Downregulation of Gα12 expression was confirmed at both the mRNA and protein levels via RT-PCR and western blotting, respectively. As shown in Fig. 1C and D, Gα12 siRNA markedly increased the serum withdrawal-induced apoptosis of HUVECs by up to 313% when compared with control siRNA-transfected cells, as shown by the level of cleaved caspase-3 expression. These data were confirmed by the TUNEL assay. The knockdown of Gα12 expression also augmented the serum withdrawal-induced p38 activation, which is known to mediate the apoptosis of numerous cells, including endothelial cells. These results show that serum deprivation induced the apoptosis of HUVECs and that apoptosis was augmented by Gα12 deficiency in HUVECs. These findings suggest that Gα12 might protect vascular endothelial cells from endothelial damage such as apoptotic cell death.
To investigate how Gα12 regulates serum withdrawal-induced apoptosis, we attempted to determine whether Gα12 is involved in the regulation of miRNA expression. HUVECs were transfected with Gα12 siRNA, and the expression of the miRNAs miR-17-3, miR-31, miR-155, and miR-191, which have been reported to be involved in the regulation of endothelial damage including inflammation and apoptosis, was measured.1011 Gα12 siRNA reduced the expression of miR-17-3, miR-31, and miR-191. This finding suggests that Gα12 can modulate the expression of miRNAs in endothelial cells. Gα12 siRNA markedly suppressed miR-155 expression, which is known to be involved in the regulation of endothelial cell apoptosis (Fig. 2). To examine the relationship between miR-155 and apoptosis in HUVECs, HUVECs were cultured in serum-free conditions for 6 h, and the expression of miR-155 was measured. Serum withdrawal significantly suppressed the expression of miR-155 (Fig. 3A). To confirm the effects of miR-155 on HUVEC apoptosis, HUVECs were transfected with an miR-155 mimic or miR-155 inhibitor, and the miR-155 level was measured and compared with that in control-transfected cells (Fig. 3B). The miR-155 mimic inhibited the serum withdrawal-induced apoptosis, and the miR-155 inhibitor increased HUVEC apoptosis (Fig. 3C and D). These results suggest that miR-155 is involved in the regulation of HUVEC apoptosis. To examine the role of miR-155 in the regulation of apoptosis in the Gα12-deficient cells, HUVECs were cotransfected with Gα12 siRNA and miR-155 mimic and then deprived of serum for 6 h. The miR-155 mimic significantly inhibited the increased apoptosis and p38 phosphorylation of Gα12-deficient HUVECs (Fig. 3E), indicating that miR-155 acts downstream of Gα12 in protecting HUVECs against apoptosis. These results suggest that Gα12 modulates the apoptosis of vascular endothelial cells by regulating the expression of miR-155.
In this study, we investigated the role of Gα12 in the regulation of vascular endothelial cell apoptosis and its underlying mechanisms. We found that Gα12 protected HUVECs against serum withdrawal-induced apoptosis by inhibiting the suppression of miR-155 expression. This finding was supported by results showing that Gα12 siRNA increased the serum withdrawal-induced apoptosis of HUVECs. Additionally, Gα12 siRNA markedly suppressed the expression of miR-155, and the restoration of miR-155 by transfection with a miR-155 mimic abolished the increased apoptosis of Gα12-deficient HUVECs. These results indicate that Gα12 can inhibit the apoptosis of endothelial cells by retaining the expression of miR-155 against the cell death signal.
As signal transducers, G proteins are involved in the modulation of cellular responses, including proliferation, differentiation, migration, and apoptosis. Several studies have shown that Gα12 is involved in the regulation of proliferation and migration. Gα12 mediates the lysophosphatidic acid-induced proliferation of ovarian cancer cells and simulates the expression of genes that promote cell growth in NIH3T3 cells.1213 Overexpression of the constitutively active mutant of Gα12 promotes invasion of breast and prostate cancer cells1415 and migration of VSMCs by inducing CYR61 expression.16 Gα12 has also been reported to exert a proapoptotic effect in epithelial cells. Overexpression of the constitutively active mutant of Gα12 stimulates the apoptosis of Madin-Darby canine kidney cells via c-Jun N-terminal kinase activation and also induces COS-7 cell apoptosis via the MAPK pathway, including apoptosissignal regulating kinase 1 and MAPK kinase kinase 1.1718 In contrast, we found that blockade of endogenous Gα12 expression augmented the serum withdrawal-induced apoptosis of HUVECs. This finding indicated that Gα12 inhibited the apoptosis of HUVECs and protected them against cellular stressinduced endothelial dysfunction, which suggests that the role of Gα12 in the modulation of apoptosis might exhibit cell-type specificity.
Vascular endothelial cells form the lining of the inner surface of blood vessels. Injury or damage to endothelial cells by inflammatory cytokines, oxidative stress, modified lipoproteins, or apoptosis initiates the pathogenesis of vascular diseases such as atherosclerosis. Apoptosis of endothelial cells is regarded as an initial step in the development of atherosclerosis. Apoptotic cells are found in atherosclerotic plaque and are thought to play a role in the formation of coronary thrombotic atherosclerotic plaque.1920 Thus, apoptosis of endothelial cells could reflect endothelial dysfunction caused by endothelial damage. If so, regulating the apoptosis of endothelial cells may provide a way to prevent or inhibit the pathogenesis of atherosclerosis. Little is known about the role of Gα12 in the regulation of endothelial cell death. Thus, we were interested in studying the role of Gα12 in the modulation of endothelial cell apoptosis. Our findings suggest that Gα12 plays a crucial role in the vascular system by modulating endothelial damage.
This study also demonstrated the mechanisms by which Gα12 regulates the apoptosis of vascular endothelial cells. Serum withdrawal caused apoptosis and the repression of miR-155, and Gα12 siRNA markedly suppressed miR-155 expression, which caused HUVECs to become more susceptible to apoptosis induced by serum deprivation. Using an miR-155 mimic, we found that increased miR-155 expression inhibited the serum deprivation-induced apoptosis of HUVECs and prevented the increased apoptosis caused by blockade of Gα12 expression. These findings suggest that the repression of miR-155 is responsible for the increased susceptibility of HUVECs to apoptosis. Taken together, our data support the roles of Gα12 and miR-155 in protecting vascular endothelial cells from apoptotic cell death. MiR-155 has been found to be involved in the development of cancer and in the pathogenesis of cardiovascular diseases including atherosclerosis. MiR-155 stimulates cell proliferation and migration and inhibits apoptosis of renal cancer cells, which suggests that miR-155 contributes to the oncogenesis of tumor cells.21 However, the role of miR-155 in the pathogenesis of atherosclerosis is debated. Silencing miR-155 increases inflammation in macrophages and their lipid uptake, indicating that miR-155 is antiatherogenic. Another study showed that miR-155 exerts proatherogenic effects by repressing Bcl6 in macrophages.2223 MiR-155 is enriched in endothelial cells, and the effects of miR-155 are thought to be antiatherogenic in endothelial cells, a hypothesis that is supported by the finding that miR-155 inhibits angiotensin II (Ang II)-induced inflammation, migration, and apoptosis of HUVECs by targeting the Ang II type 1 receptor.1124 Consistent with this, our study showed that overexpression of miR-155 inhibited serum withdrawal-induced apoptosis of HUVECs and prevented the increased apoptosis mediated by Gα12 knockdown, indicating that miR-155 acts downstream of Gα12 to play a protective role against apoptotic cell death. Although Gα12 siRNA markedly reduced the expression of miR-155, Gα12 siRNA by itself did not induce detectable apoptosis, and miR-155 inhibition by itself could not induce apoptosis. The miR-155 inhibitor induced apoptosis only when HUVECs were exposed to an apoptotic stimulus, as shown in Fig. 3D. This indicates that repression of miR-155 alone is not sufficient to induce apoptosis, although it causes HUVECs to become more susceptible to apoptosis, as shown by the increased apoptosis compared with HUVECs that retained the expression of miR-155.
From these results, we conclude that Gα12 protects HUVECs against serum withdrawal-induced apoptosis by regulating the expression of miR-155. This suggests that endothelial apoptosis can be modulated by the Gα12 signaling pathway via the regulation of miRNAs. Novel strategies to protect endothelial cells from endothelial dysfunction by regulating the Gα12 signaling pathway may have potential in preventing cardiovascular diseases.
Figures and Tables
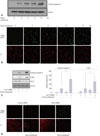 | Fig. 1Gα12 inhibits serum withdrawal-induced apoptosis of HUVECs. (A and B) Effect of serum deprivation on HUVEC apoptosis. HUVECs were deprived of serum for the indicated times, and apoptosis was assessed via immunoblot analysis with antibody to cleaved caspase-3 and a TUNEL assay. (C and D) Effect of Gα12 siRNA on serum withdrawal-induced apoptosis. HUVECs were transfected with Gα12 siRNA. These cells were cultured 48 h after transfection in the serum-free condition for 6 h and were analyzed for apoptosis via immunoblotting with antibodies to cleaved caspase-3 and phosphorylated p38 and the TUNEL assay. The blots are representative of at least three independent experiments, and the histograms show the average and standard deviation. *p<0.05 compared with control cells. Gα12, alpha 12 subunit of G protein; HUVEC, human umbilical vein endothelial cell; siRNA, small-interfering RNA; PI, propidium iodide. |
 | Fig. 2Gα12 alters the expression of miRNAs in HUVECs. HUVECs were transfected with Gα12 siRNA, and the expression of miRNAs was assessed via quantitative RT-PCR. The RNA was isolated, and the levels of miR-17-3, miR-31, miR-155, and miR-191 were measured. The histograms show the average and standard deviation of three independent experiments. *p<0.05, **p<0.005 compared with control cells. Gα12, alpha 12 subunit of G protein; HUVEC, human umbilical vein endothelial cell; siRNA, small-interfering RNA; miRNA, microRNA; RT-PCR, realtime PCR. |
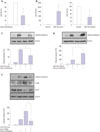 | Fig. 3Gα12 modulates the apoptosis of HUVECs by regulating the level of miR-155. (A) Effect of serum deprivation on the expression of miR-155. HUVECs were deprived of serum for 6 h, and the expression of miR-155 was determined by quantitative RT-PCR. (B) Regulation of miR-155 level by a mimic or inhibitor of miR-155. HUVECs were transfected with a mimic or inhibitor of miR-155, and the expression of miR-155 was determined by quantitative RT-PCR. (C and D) Effects of a mimic and inhibitor of miR-155 on serum withdrawal-induced apoptosis. HUVECs were transfected with the mimic or inhibitor for miR-155 and then deprived of serum for 6 h, and the expression of cleaved caspase-3 was measured. (E) Effect of miR-155 mimic on apoptosis in Gα12-deficient HUVECs. HUVECs were cotransfected with Gα12 siRNA and miR-155 mimic and cultured in the serum-free condition for 6 h, and cleaved caspase-3 and phosphorylated p38 were assessed. The blots are representative of at least three independent experiments, and the histograms show the average and standard deviation. *p<0.05 compared with control cells. Gα12, alpha 12 subunit of G protein; HUVEC, human umbilical vein endothelial cell; RT-PCR, real-time PCR; siRNA, small-interfering RNA. |
ACKNOWLEDGEMENTS
This work was supported by a faculty grant from Yonsei University College of Medicine (No. 2014-32-0049) and a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare Affairs (HI08C2149), Republic of Korea.
References
2. Xu N, Bradley L, Ambdukar I, Gutkind JS. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993; 90:6741–6745.

3. Gan CP, Patel V, Mikelis CM, Zain RB, Molinolo AA, Abraham MT, et al. Heterotrimeric G-protein alpha-12 (Gα12) subunit promotes oral cancer metastasis. Oncotarget. 2014; 5:9626–9640.

4. Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005; 435:834–838.

5. Quintavalle M, Condorelli G, Elia L. Arterial remodeling and atherosclerosis: miRNAs involvement. Vascul Pharmacol. 2011; 55:106–110.

6. Ma X, Ma C, Zheng X. MicroRNA-155 in the pathogenesis of atherosclerosis: a conflicting role. Heart Lung Circ. 2013; 22:811–818.

7. Yanamadala V, Negoro H, Denker BM. Heterotrimeric G proteins and apoptosis: intersecting signaling pathways leading to context dependent phenotypes. Curr Mol Med. 2009; 9:527–545.

8. Seo M, Cho CH, Lee YI, Shin EY, Park D, Bae CD, et al. Cdc42-dependent mediation of UV-induced p38 activation by G protein betagamma subunits. J Biol Chem. 2004; 279:17366–17375.

9. Ro S, Park C, Jin J, Sanders KM, Yan W. A PCR-based method for detection and quantification of small RNAs. Biochem Biophys Res Commun. 2006; 351:756–763.

10. Suárez Y, Wang C, Manes TD, Pober JS. Cutting edge: TNF-induced microRNAs regulate TNF-induced expression of E-selectin and intercellular adhesion molecule-1 on human endothelial cells: feedback control of inflammation. J Immunol. 2010; 184:21–25.

11. Liu T, Shen D, Xing S, Chen J, Yu Z, Wang J, et al. Attenuation of exogenous angiotensin II stress-induced damage and apoptosis in human vascular endothelial cells via microRNA-155 expression. Int J Mol Med. 2013; 31:188–196.

12. Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN. Lysophosphatidic Acid Stimulates the Proliferation of Ovarian Cancer Cells via the gep Proto-Oncogene Gα(12). Genes Cancer. 2011; 2:563–575.

13. Kumar RN, Radhika V, Audigé V, Rane SG, Dhanasekaran N. Proliferation-specific genes activated by Galpha(12): a role for PDGFRalpha and JAK3 in Galpha(12)-mediated cell proliferation. Cell Biochem Biophys. 2004; 41:63–73.
14. Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem. 2006; 281:26483–26490.

15. Kelly P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2006; 103:8173–8178.

16. Kim YM, Lim SC, Han CY, Kay HY, Cho IJ, Ki SH, et al. G(alpha)12/13 induction of CYR61 in association with arteriosclerotic intimal hyperplasia: effect of sphingosine-1-phosphate. Arterioscler Thromb Vasc Biol. 2011; 31:861–869.

17. Yanamadala V, Negoro H, Gunaratnam L, Kong T, Denker BM. Galpha12 stimulates apoptosis in epithelial cells through JNK1-mediated Bcl-2 degradation and up-regulation of IkappaBalpha. J Biol Chem. 2007; 282:24352–24363.

18. Berestetskaya YV, Faure MP, Ichijo H, Voyno-Yasenetskaya TA. Regulation of apoptosis by alpha-subunits of G12 and G13 proteins via apoptosis signal-regulating kinase-1. J Biol Chem. 1998; 273:27816–27823.

19. Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001; 33:1673–1690.

20. Xu F, Sun Y, Chen Y, Sun Y, Li R, Liu C, et al. Endothelial cell apoptosis is responsible for the formation of coronary thrombotic atherosclerotic plaques. Tohoku J Exp Med. 2009; 218:25–33.

21. Li S, Chen T, Zhong Z, Wang Y, Li Y, Zhao X. microRNA-155 silencing inhibits proliferation and migration and induces apoptosis by upregulating BACH1 in renal cancer cells. Mol Med Rep. 2012; 5:949–954.

22. Huang RS, Hu GQ, Lin B, Lin ZY, Sun CC. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J Investig Med. 2010; 58:961–967.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download