Abstract
Behçet's disease (BD) is a multi-systemic inflammatory disorder of an unknown etiology and shows a chronic recurrent clinical course. When the disease involves the alimentary tract, it is called intestinal BD because of its clinical importance. Intestinal BD is more frequently reported in East Asian countries than in Western or Middle Eastern countries. While any part of the gastrointestinal tract can be involved, the most common location of intestinal BD is the ileocecal area. A few, large, deep ulcerations with discrete border are characteristic endoscopic findings of intestinal BD. Currently, there is no single gold standard test or pathognomonic finding of intestinal BD. However, recently developed novel diagnostic criteria and a disease activity index have helped in assessing intestinal BD. As intestinal BD shares a lot of characteristics with inflammatory bowel disease, including genetic background, clinical manifestations, and therapeutic strategies, distinguishing between the two diseases in clinical practice is quite difficult. However, biologic agents such as anti-tumor necrosis factor α antibody shows a considerable efficacy similar to inflammatory bowel disease cases. It is important to distinguish and treat those two disease entities separately from the standpoint of precise medicine. Clinicians should require comprehensive knowledge regarding the similarities and differences between intestinal BD and inflammatory bowel disease for making an accurate clinical decision.
Behcet's disease (BD) is a chronic relapsing multi-systemic inflammatory disease of an unknown etiology characterized by repeated oral and genital ulcerations, ocular lesions, skin manifestations, arthritis, vasculitis, and gastrointestinal involvement. 123 Intestinal BD occurs in 3-60% of patients with BD.234 East Asian countries such as Korea and Japan tend to have a higher frequency of gastrointestinal involvement of BD than Western or Middle Eastern countries.5 Although clinical manifestations of intestinal BD vary widely from mild abdominal pain to bowel perforation or massive hemorrhage, gastrointestinal involvement of BD often predicts poor treatment response and unfavorable prognosis of the affected patients.567
Intestinal BD and inflammatory bowel disease (IBD) share a considerable number of genetic backgrounds, pathogenesis, and clinical features. Moreover, current therapeutic strategies for intestinal BD have many similarities to those of IBD. Some experts classify the two diseases as the same category of a single disease or as different spectrums of the same disease; others regard them as totally different diseases. In this review, we will discuss the similarities and differences between intestinal BD and IBD (Table 1).
The exact etiology and pathophysiology of BD remains unclear. Nevertheless, similar to IBD, both genetic and environmental factors might contribute to the development of BD.8 Classically, an human leukocyte antigen (HLA)-B51 allele has been thought to be the most important genetic factor of BD.9 Although it is still unclear whether this locus independently acts as the fundamental cause of BD development, studies of BD report that the prevalence of the HLA-B51 allele is much higher in patients with BD than in unaffected populations.310 Genome-wide association studies (GWAS) from Japan and Turkey have also confirmed HLA-B51 as a susceptible locus for BD.1112 Another susceptibility locus, MHC class I related gene A (MICA), has been evaluated in several studies.81314 However, an independent contribution for these loci has not proven to be easy to confirm due to the strong linkage disequilibrium with HLA-B51.15 HLA-B51 or MICA has not been shown to be associated with IBD susceptibility.
Recent studies report an association for BD with interleukin (IL) 10 and the IL23R-IL12RB2 loci.1112 Decreased mRNA expression and low protein production was correlated with BD expression. Similarly, IL10 or IL23R variants were also observed in IBD patients, suggesting that the two diseases have similar genetic backgrounds and pathogenesis.16 However, polymorphisms of genetic variants of IL10 and IL23R in patients with intestinal BD were not associated with those of IBD.17 The IL10-1082AA and -819T genotype were associated with BD, while IL10-819CT and -592CA polymorphisms were related with ulcerative colitis. IL10-1082GA was not associated with IBD.1819 IBD is known to exhibit an association with variants in IL23R, IL12B, and TYK2, while BD is associated with the intergenic region between IL23R and IL12RB2.20 In the Korean population, haplotypes of IL17A demonstrated a risk of developing intestinal BD, while those of IL23R were associated with disease protection.21
Close overlap of genetic variants provides considerable explanation about phenotypic and clinical similarities between intestinal BD and IBD. Despite many parallels between the two diseases, detailed distinctions regarding genetics have been steadily traced. Therefore, further studies are needed to discover the exact genetic contributions for each disease.
Although BD shows familial aggregation and a genetic background, environmental factors also contribute to triggering inflammation. Increased Th1, Th17, CD4+ and CD8+ T cell, and γδ+ T cell activities were found both in the serum or inflamed tissues of BD patients,1422232425 which suggests that innate and adaptive immunity act together to initiate BD. Similar to other autoimmune disorders, BD shows Th1-type cytokine profiles. IL-2 and interferon (INF)-γ producing T cells were increased in patients with active BD, while IL-4 producing T cells were lower than in controls.26 IL-12 and tumor necrosis factor (TNF)-α levels were also increased in BD.262728 However, contrary to typical autoimmune disorders, CD5+CD19+ B cell levels were low, and autoimmune markers such as antinuclear antibodies were negative.14 The immunologic pathogenesis of IBD is summarized as exhibiting dysfunctions of the epithelial barrier, innate immune cells, and adaptive T cells.29 In patients with IBD, innate (macrophage, neutrophil) and acquired (T and B cell) immune responses are activated.30 Most pro-inflammatory cytokines involved in innate immune system are activated in both Crohn's disease and ulcerative colitis. As in BD, Th1 and Th17 related cytokines, such as IL-12, IL-23, and IL-27, are also up-regulated in Crohn's disease.31 Moreover, Th1 cell-related cytokines, such as IFN-γ and IL-2, are also increased in Crohn's disease.32 In patients with ulcerative colitis, however, T cells from the lamina propria highly produce IL-5 and IL-13, which are regarded as Th2-cell related cytokines.32 So far, relatively less research has been conducted on the immunology of BD.
Serum anti-herpes simplex virus (HSV)-1 antibodies in patients with BD were significantly higher than those in controls, 33 and HSV DNA was found in the genital and intestinal ulcers of patients.34 Sohn, et al.3536 developed and reported a BD murine model through HSV inoculation in ICR mice. However, antiviral therapy seems controversial for the treatment of BD. Though other viruses, including hepatitis C, parvovirus B19, cytomegalovirus, Epstein-Barr virus, and varicella zoster virus, may contribute to BD, results are inconsistent.37 The exact role of viruses in the pathogenesis of IBD is not clearly demonstrated.383940 Cytomegalovirus is frequently related with severe or steroid refractory ulcerative colitis.41 Cytomegalovirus seems to be associated with steroid refractoriness. However, the causal relationships between ulcerative colitis and cytomegalovirus are not clear.4142
Generally, BD starts from oral ulcerations. Therefore, bacteria from normal flora of the oral mucosa have been evaluated as causative organisms. A number of Streptococcus species have been implicated. Streptococcus sanguis and its antibodies are repeatedly detected in the oral mucosa and sera of patients with BD.43 Streptococcus sanguis-related antigen (KTH-1) stimulates IL-6 and INF-γ production in patients with BD.44 Streptococcus sanguis antigen has a homology with a cellular membrane protein called heat shock protein (HSP). Mycobacterial HSP-65 and human HSP-60 share over 50% of sequence homology,33 and HSPs have been found to stimulate γδ+ T cells in BD patients.4546 Therefore, researchers have postulated that specific bacterial antigens induce mucosal HSP against bacterial stimulation and concurrently activate T cells against intestinal mucosa in BD patients. However, the exact role of those antigens as an inducer of primary autoimmunity remains obscure.
In the same manner, intestinal microbiota may play an important role in IBD development. Dysbiosis of conventional microbiota, pathogenic stimulation of functionally altered commensal bacteria, host genetic defects in containing microbiota, and defective host immune regulation are generally accepted explanations of the pathogenesis of IBD.47 In a study comparing mucosal flora between 40 controls and 305 patients with an inflamed bowel, including 54 Crohn's disease and 119 ulcerative colitis, concentrations of mucosa-associated bacteria were higher in patients with intestinal inflammation than in controls.48 Recent studies have revealed that the dysbiosis of intestinal microbiota in patients with IBD is characterized by reduction in diversity, prominent depletion of Bacteroidetes and Firmicutes including Clostridium XIVa/IV, and growth of Actinobacteria and Proteobacteria.495051 Mycobacterium avium subspecies paratuberculosis, adherent-invasive Escherichia coli, Clostridium difficile toxin A and enterotoxigenic Bacteroides fragilis have been thought to be possible pathogens of IBD.525354555657 However, the exact relationships between these bacteria and induction of IBD have not been confirmed due to differences among individuals and considerable alterations of intestinal microbiota.
Abdominal pain, diarrhea, melena, and hematochezia can occur in patients with intestinal BD.585960 Clinical signs such as abdominal tenderness or a palpable mass on the affected area, fever, and weight loss are also noted.58 Gastrointestinal manifestations of BD usually develop 4.5-6 years after the onset of oral ulcerations.5 Sometimes, however, intestinal lesions can precede extra-intestinal manifestations.61 Theoretically, there are two forms of intestinal lesions: one is mucosal inflammations and ulcerations by neutrophilic phlebitis. The other involves ischemic damage from vasculitis.5 The most frequently involved location is the ileocecal area. However, any part of the alimentary tract and extra-intestinal organs, such as liver, pancreas, or spleen, can be affected. While anal complication is frequently observed in patients with Crohn's disease, rectal or anorectal involvement of intestinal BD is rare.62 Also, intestinal complications such as stricture, fistula, and abscess formation are more frequent in patients with Crohn's disease due to its transmural nature of inflammation.62 Oral ulceration is usually considered separately, because oral ulceration plays a major role in diagnosing BD.3 Although the frequencies of gastrointestinal involvement in patients with BD have been reported variously, depending on geographical location, its actual incidence might be higher than study results, because of the possible presence of asymptomatic lesions. A Chinese study evaluating screening colonoscopies of systemic BD patients found 35.1% had gastrointestinal lesions. Of them, four of 18 patients with active ulcerations showed no gastrointestinal symptoms.63 Importantly, extra-intestinal manifestations of BD, such as oral and genital ulcerations, ocular and join involvement, and skin lesions, are all potentially experienced in the course of IBD. Thus, differential diagnosis between BD and IBD remains a challenge.
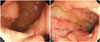
Typical ulcerations of intestinal BD are described as a single or few, large, discrete, and round or oval shaped ulcerations in the ileocecal area.559 However, various ulcerations from small aphthous ulcerations to multiple irregular shaped ulcerations can be observed. As intestinal BD and Crohn's disease share similar clinical courses, extra-intestinal manifestations, and non-specific gastrointestinal symptoms, it is often difficult to differentiate between the two.3 Lee, et al.64 compared colonoscopic findings of 115 intestinal BD and 135 Crohn's disease patients. Multivariate analysis revealed that round shape, fewer number (≤5), focal distribution, and absence of aphthous and cobble stone appearance were independent discriminating factors of intestinal BD. Furthermore, they proposed a diagnostic algorithm using classification analysis (Fig. 1). Dominant colonoscopic findings of intestinal BD also can be used. A study suggested a novel diagnostic criteria for intestinal BD using systemic and colonoscopic features of BD. Investigators regarded five or fewer lesions, oval shape, deep penetrating, discrete border, and ileocecal location as typical ulcerations. Overall, the positive predictive value and accuracy of the diagnostic algorithm were 86.1% and 91.1%, respectively.65 Kim, et al. classified macroscopic findings of intestinal BD ulcerations into volcano, geographic, and aphthous types. Volcano type ulceration (well-demarcated deeply penetrating ulcers with nodular margins, converging folds, or pseudopolyps) was associated with poor prognosis.6667 Yim, et al.68 reported complete resolution of inflammation by macro- and microscopic evaluation, so called "mucosal healing," to be significantly associated with favorable clinical course, which is consistent with Crohn's disease cases. Identifying active intestinal ulcerations during clinical remission was independently related with clinical relapse. Classical endoscopic findings of Crohn's disease in colonoscopic examination include discontinuous chronic mucosal inflammation, aphthoid ulcerations, longitudinal ulcerations, and cobblestone appearance with normal surrounding mucosa.69 Skipped inflammatory lesions with normal intervening mucosa are frequently observed in Crohn's disease, similar to those of intestinal BD. However, distribution patterns in patients with Crohn's disease are more diffuse than in patients with intestinal BD (Fig. 2).64
There are no pathognomonic histologic findings regarding intestinal BD. Vasculitis affecting small veins and venules are commonly accepted features.70 However, these findings are not consistently observed in affected organs. Histology from esophageal biopsy, for example, often shows non-specific inflammation (lymphocytic or neutrophilic infiltrations) rather than vasculitis.71 Generally, absence of non-caseating granuloma implies intestinal BD rather than Crohn's disease. However, non-caseating granulomas are observed in only 15-36% of patients with Crohn's disease.72 Other histologic characters of Crohn's disease, such as transmural inflammation, chronicity, and focality, are also able to be observed in intestinal BD. Normal circumferential mucosa surrounding a large ulceration is one of the characteristic histologies of intestinal BD. A study comparing histology from rectal biopsy between 75 patients with BD and 14 ulcerative colitis revealed that destruction of surface epithelium, polymorphonuclear infiltration, loss of goblet cells, and loss of crypts are more frequently observed in ulcerative colitis than in BD.73
The Korean IBD Study Group developed a novel tool for assessing disease activity in patients with intestinal BD.74 As IBD exhibits an unpredictable wax-and-wane clinical course and various manifestations, disease activity should be monitored by an organized measuring method. Investigators have developed and applied several validated disease activity indices for each Crohn's disease and ulcerative colitis.7576777879 Before developing disease activity index for intestinal Behcet's disease (DAIBD), some clinicians had used disease activity index of Crohn's disease for evaluating intestinal BD activity. DAIBD includes the general condition of a patient, extra-intestinal manifestations, intestinal complications, symptoms and signs, and stool frequency. Whereas taking antidiarrheal agents and the presence of complications are highly weighted in Crohn's disease activity index (CDAI), DAIBD considers the general condition of patient and abdominal pain more importantly. Based on the cumulative score of each item, disease activity is categorized into "severe," "moderate," "mild," and "quiescent". DAIBD showed much higher responsiveness than the CDAI (r=0.812 vs. r=0.645, respectively). However, a recent study of the same group revealed that DAIBD was not highly associated with endoscopic severity, which is similar to Crohn's disease cases.80
The rate of anti-Saccharomyces cerevisiae antibodies (ASCA) detection is remarkably higher in patients with BD, especially in patients with gastrointestinal involvement, than in controls. 81 In a study evaluating ASCA and the clinical course of intestinal BD patients, similar to those of Crohn's disease, ASCA was positive in 44.3% of intestinal BD patients. ASCA positive patients were also more likely to receive surgical treatment.82 The results further implied a similarity between Crohn's disease and intestinal BD. However, atypical or perinuclear anti-neutrophil cytoplasmic autoantibody was not shown to be related with intestinal BD.83 Studies regarding anti-endothelial cell antibody (AECA) revealed a high prevalence of AECA in systemic vasculitis including BD.84 α-enolase protein is the target of AECA.8586 Serum soluble triggering receptor expressed in myeloid cells-1 was significantly associated with DAIBD in intestinal BD patients, although not with C-reactive protein (CRP) or erythrocyte sedimentation rate.87
There is a lack of randomized prospective studies regarding the treatment of intestinal BD.8889 Traditionally, therapeutic implications of intestinal BD have been similar to those of Crohn's disease.6089 There is a controversy about the therapeutic effects of 5-amino-salicylates (5-ASA)/sulfasalazine, which is routinely used in patients with IBD.909192 In a retrospective cohort study investigating 143 patients with intestinal BD receiving 5-ASA/sulfasalazine alone for maintaining remission, cumulative relapse rates at 1, 3, 5, and 10 years after remission were 8.1%, 22.6%, 31.2%, and 46.7%, respectively. Younger age at diagnosis (<35 years), higher serum CRP level (1.5 mg/dL), and greater DAIBD score (≥60) independently predicted clinical relapse.93
Systemic corticosteroids are available for inducing remission in patients with moderate to severe disease or when treatment with 5-ASA/sulfasalazine fails.94 Starting 0.5-1 mg/kg of prednisolone or its equivalent and rapid tapering strategies are prevalent, similar to IBD treatment.95 The dose of corticosteroids should be adapted according to the severity of the disease. 3 In a retrospective cohort study, systemic corticosteroid therapy (mean starting dose, 0.58 mg/kg) in 54 patients with active intestinal BD showed 46.3% complete remission, 42.6% partial remission, and 11.1% no response after a month from treatment. After one year, however, prolonged responses were found in 4 only 8.1% of the cohort, while 35.2% of patients showed corticosteroid dependency.96 Compared with a similar study evaluating clinical outcomes in patients with Crohn's disease receiving oral prednisolone, prolonged response (56.6%) and corticosteroid dependency (24.1%) at a year after receiving treatment showed a better clinical course in patients with Crohn's disease than in intestinal BD.97
Thiopurines or azathioprine/6-mercaptopurine (AZA/6-MP) are indicated in patients who show corticosteroid dependency or resistance.95 In a double-blind, randomized, placebo-controlled trial, AZA had a beneficial effect in controlling BD including eye and extra-ocular diseases.98 In patients with intestinal BD, Jung, et al.99 reported cumulative relapse rates of 5.8%, 28.7%, 43.7%, and 51.7% at 1, 2, 3, and 5 years after remission among patients who received AZA/6-MP for remission maintenance, respectively. Multivariate analysis showed a young age at diagnosis (<25 years) and lower serum hemoglobin level (<11 g/dL) to be independent predictors of relapse. Similarly, a retrospective study revealed cumulative relapse rates of 18.0% and 49.2% in patients with Crohn's disease who were treated continuously with AZA/6-MP to maintain clinical remission after 1 and 3 years of treatment, respectively. Independent predictive factors of relapse during AZA/6-MP treatment were younger age at treatment and increased serum CRP level at remission status.100
Thalidomide (100-300 mg/day) was found to be effective on treating oral and genital ulcerations, as well as follicular lesions, of BD in a randomized, double-blind, placebo-controlled study.101 Another small case series reported that thalidomide (1-3 mg/kg) was able to replace steroid therapy without serious complications in juvenile-onset intestinal BD.102 A small study investigating thalidomide in patients with IBD reported clinical response rates of 83.3% and 100.0% after 12 weeks of 100-400 mg per day thalidomide treatment in patients with Crohn's disease and ulcerative colitis, respectively.103 However, continuous vigilance for long-term side effects of thalidomide is warranted.
Monoclonal antibodies to TNF-α, including infliximab (IFX) and adalimumab (ADA), are important biological agents for treating IBD.30 After several reports104105106107108 on therapeutic effects of anti-TNF-α in patients with intestinal BD, accumulation of evidence on the therapeutic impacts of anti-TNF-α treatment has increased. Nevertheless, there is a need to investigate the impact of anti-TNF-α treatment on cumulative surgery rates and post-operative recurrence. Currently, there is no large scale, randomized trial of anti-TNF-α agents in patients with intestinal BD. However, several case series reported favorable results of IFX on induction and maintain remission of intestinal BD.109110111 A retrospective multicenter study in Korea, evaluating 28 cases of patients with intestinal BD treating with IFX who were refractory to conventional medical treatments, reported a clinical response rate of 64.8% at 4 weeks after treatment. 112 Older age (≥40 years), female gender, longer disease duration (≥5 years), using concomitant immunomodulators, and achieving clinical remission were independently associated with sustained response. A prospective, multicenter, randomized, double-blind, placebo-controlled study (ACCENT I) revealed that IFX induced clinical remission (CDAI<150) in 58% of patients with Crohn's disease.113 Also, patients who received scheduled IFX were more likely to sustain clinical remission than those who did not.114 The impacts of maintenance therapy with IFX were consistently confirmed, even in patients with fistulizing Crohn's disease who responded to induction therapy (ACCENT II).115
Recently, the clinical efficacy of ADA was also ascertained by case reports116117118 and a small randomized trial119 similar to IFX. A phase III, multicenter, open-label, uncontrolled study investigating the efficacy and safety of ADA for treating 20 active intestinal BD patients who were refractory to conventional therapy in Japan revealed complete remission in 20% at week 52 with similar rates of adverse events as in other clinical trials regarding ADA.119 Two randomized, double-blind, placebo-controlled studies similarly evaluated the efficacy of remission induction and maintenance of ADA in patients with Crohn's disease. A study investigating 299 patients with moderate-to-severe Crohn's disease naive to anti-TNF-α agents who were randomized to receive ADA (40/20 mg, 80/40 mg, or 160/80 mg) or placebo at weeks 0 and 2 showed remission rates of 18%, 24%, and 36% of (CDAI<150) at week 4, respectively. 120 A follow-up study revealed that 40 mg of ADA injection every other week or weekly showed significantly higher remission rates (79% and 83%, respectively) at week 56 than placebo (44%, p<0.05).121 Another study evaluating 854 patients with moderate to severe Crohn's disease who received 80 mg of ADA at week 0 and 40 mg at week 2 showed clinical response in 58.4% (drop in CDAI of 70 points) at week 4. Also, 40% of the 40 mg every other week group, 47% of the 40 mg weekly group, and 17% of the placebo group were in remission at week 26.122 A consensus statement of anti-TNF-α therapy in patients with intestinal BD by the Japanese group proposed its indication as a standard therapy for intestinal BD.123 However, a large scale, randomized, prospective trial is needed for the establishment of the long-term efficacy of anti-TNF-α therapy.
Surgical resection of an affected bowel is considered when patients with intestinal BD are refractory to medical treatment or serious complications, such as bowel perforation or severe bleeding, cannot be controlled by conservative treatment.124 Lee, et al.125 reported peritonitis due to multiple perforations was the most common indication of surgery in patients with intestinal BD, following gastrointestinal bleeding, entero-cutaneous fistula, and intractable pain with recurrence. Bowel perforation is one of the most disastrous complications of intestinal BD. A retrospective analysis regarding free bowel wall perforation in 129 patients with intestinal BD reported that 33 (25.6%) of patients experienced bowel perforation and consecutive surgery. Of them, 14 (42.4%) showed post-operative recurrence and 11 (33.3%) underwent re-operation. Younger age at diagnosis (≤25 years), experience of prior laparotomy, and volcano-shaped ulceration predicted bowel perforation independently.126
Compared to Crohn's disease, intestinal BD shows similar cumulative surgery rates (29.4% and 36.0% in Crohn's disease vs. 31.6% and 44.4% in intestinal BD at five and ten years, respectively: p=0.287).124 Naganuma, et al.127 reported that small bowel involvement and ocular lesion in patients with intestinal BD were significantly associated with requiring surgery. Likewise, pouchitis after ileal pouch-anal anastomosis in patients with IBD was highly related with extra-intestinal manifestations. 128 However, intestinal BD surgery shows distinct features. While the extent of bowel resection should be restricted in patients with Crohn's disease,129 that of intestinal BD is still controversial. Traditionally, resecting a sufficient margin including normal intestine was recommended in surgery of intestinal BD.6130131 However, more recent investigators have asserted minimal resection, which is restricted in affected bowel, because resection length was not related with post-operative recurrence in patients with intestinal BD.58124132 Due to relatively high rates of complications at anastomosis sites, including leakage, perforation, and fistula formation, in intestinal BD surgery, bowel diversion (stoma formation) is suggested by several investigators.133 Especially, because pathergy is often seen with BD patients, ulcerations at the site of surgical incision can develop.134
A study investigating the clinical course of intestinal BD during the first 5 years after diagnosis reported that 74.6% of patients were in remission or showed mild disease activity at years 5. The independent predictor of severe clinical course was higher DAIBD (≥40) at diagnosis (OR: 6.2, 95% CI: 1.1-33.5, p=0.035).135 The same group also compared long-term clinical outcomes between intestinal BD and Crohn's disease. The cumulative probabilities of surgery, hospital admission, and post-operative recurrence were not significantly different between intestinal BD and Crohn's disease (44.4% vs. 36.0%, 69.2% vs. 73.8%, and 66.5% vs. 79.1% at 10 years, p=0.287, 0.295, and 0.724, respectively).136 However, there were significant differences in cumulative probabilities of corticosteroid use and immunosuppressant use between intestinal BD and Crohn's disease (59.4% vs. 42.6% and 37.7% vs. 27.1%, p<0.001 and <0.001, respectively).136
The clinical manifestations of intestinal BD and IBD frequently overlap. Therefore, clinicians often encounter formidable obstacles regarding differential diagnosis at first presentation. Intestinal BD and Crohn's disease share similar genetic backgrounds, such as IL10 and the IL23R-IL12RB2 locus. Innate and adaptive immune responses activate Th1, Th17, CD4+ and CD8+ T cell, and γδ+ T cells from the stimulation of microorganisms alike. However, precise generic variants and the mechanisms of immune responses are different between the two diseases. Although clinical manifestations and endoscopic findings resemble each other, independent characteristics can be found through careful clinical evaluation. So far, treatment strategies for IBD have proven to be the most effective for controlling intestinal BD. However, in terms of understanding disease, continued efforts to out the pathogenesis and to distinguish intestinal BD from other inflammatory conditions, including Crohn's disease, must be pursued.
Figures and Tables
 | Fig. 1Diagnostic algorithm for differential diagnosis between intestinal Behçet's disease and Crohn's disease. |
Table 1
Similarities and Distinctions of Intestinal Behçet's Disease (BD) with Crohn's Disease

ACKNOWLEDGEMENTS
This research was financially supported by the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number A111428, HI13C1345, HI12C0130), by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A1008096), and by the "Kiturami" Faculty Research Assistance Program of Yonsei University College of Medicine for 2012 (6-2012-0135). This work was also supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University.
References
1. James DG. Behcet's syndrome. N Engl J Med. 1979; 301:431–432.
2. Kaklamani VG, Vaiopoulos G, Kaklamanis PG. Behçet's Disease. Semin Arthritis Rheum. 1998; 27:197–217.

4. Dilşn N, Koniçe M, Aral O, Ocal L, Inanç M, Gül A. Comparative study of the skin pathergy test with blunt and sharp needles in Behçet's disease: confirmed specificity but decreased sensitivity with sharp needles. Ann Rheum Dis. 1993; 52:823–825.
5. Bayraktar Y, Ozaslan E, Van Thiel DH. Gastrointestinal manifestations of Behcet's disease. J Clin Gastroenterol. 2000; 30:144–154.

6. Kasahara Y, Tanaka S, Nishino M, Umemura H, Shiraha S, Kuyama T. Intestinal involvement in Behçet's disease: review of 136 surgical cases in the Japanese literature. Dis Colon Rectum. 1981; 24:103–106.
7. Bradbury AW, Milne AA, Murie JA. Surgical aspects of Behçet's disease. Br J Surg. 1994; 81:1712–1721.

8. Hatemi G, Seyahi E, Fresko I, Talarico R, Hamuryudan V. Behçet's syndrome: a critical digest of the 2013-2014 literature. Clin Exp Rheumatol. 2014; 32:4 Suppl 84. S112–S122.
9. Verity DH, Marr JE, Ohno S, Wallace GR, Stanford MR. Behçet's disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens. 1999; 54:213–220.

10. Ohno S, Ohguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M. Close association of HLA-Bw51 with Behçet's disease. Arch Ophthalmol. 1982; 100:1455–1458.
11. Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet's disease susceptibility loci. Nat Genet. 2010; 42:703–706.

12. Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet's disease. Nat Genet. 2010; 42:698–702.

13. Karasneh J, Gül A, Ollier WE, Silman AJ, Worthington J. Whole-genome screening for susceptibility genes in multicase families with Behçet's disease. Arthritis Rheum. 2005; 52:1836–1842.

14. Direskeneli H. Behçet's disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001; 60:996–1002.
15. Hughes T, Coit P, Adler A, Yilmaz V, Aksu K, Düzgün N, et al. Identification of multiple independent susceptibility loci in the HLA region in Behçet's disease. Nat Genet. 2013; 45:319–324.

16. Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010; 42:1118–1125.
17. Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011; 60:1739–1753.

18. Wallace GR, Kondeatis E, Vaughan RW, Verity DH, Chen Y, Fortune F, et al. IL-10 genotype analysis in patients with Behçet's disease. Hum Immunol. 2007; 68:122–127.

19. Zou L, Wang L, Gong X, Zhao H, Jiang A, Zheng S. The association between three promoter polymorphisms of IL-10 and inflammatory bowel diseases (IBD): a meta-analysis. Autoimmunity. 2014; 47:27–39.

20. Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011; 140:1704–1712.

21. Kim ES, Kim SW, Moon CM, Park JJ, Kim TI, Kim WH, et al. Interactions between IL17A, IL23R, and STAT4 polymorphisms confer susceptibility to intestinal Behcet's disease in Korean population. Life Sci. 2012; 90:740–746.

22. Direskeneli H, Eksioglu-Demiralp E, Kibaroglu A, Yavuz S, Ergun T, Akoglu T. Oligoclonal T cell expansions in patients with Behçet's disease. Clin Exp Immunol. 1999; 117:166–170.

23. Suzuki Y, Hoshi K, Matsuda T, Mizushima Y. Increased peripheral blood gamma delta+ T cells and natural killer cells in Behçet's disease. J Rheumatol. 1992; 19:588–592.
24. Freysdottir J, Lau S, Fortune F. Gammadelta T cells in Behçet's disease (BD) and recurrent aphthous stomatitis (RAS). Clin Exp Immunol. 1999; 118:451–457.
25. Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet's disease corresponding to disease activity. Clin Exp Rheumatol. 2013; 31:3 Suppl 77. 32–40.
26. Sugi-Ikai N, Nakazawa M, Nakamura S, Ohno S, Minami M. Increased frequencies of interleukin-2- and interferon-gamma-producing T cells in patients with active Behçet's disease. Invest Ophthalmol Vis Sci. 1998; 39:996–1004.
27. Sayinalp N, Ozcebe OI, Ozdemir O, Haznedaroğlu IC, Dündar S, Kirazli S. Cytokines in Behçet's disease. J Rheumatol. 1996; 23:321–322.
28. Frassanito MA, Dammacco R, Cafforio P, Dammacco F. Th1 polarization of the immune response in Behçet's disease: a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999; 42:1967–1974.

29. Dave M, Papadakis KA, Faubion WA Jr. Immunology of inflammatory bowel disease and molecular targets for biologics. Gastroenterol Clin North Am. 2014; 43:405–424.

31. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006; 3:390–407.

33. Lehner T. The role of heat shock protein, microbial and autoimmune agents in the aetiology of Behçet's disease. Int Rev Immunol. 1997; 14:21–32.

34. Sohn S. Etiopathology of Behçet's disease: herpes simplex virus infection and animal model. Yonsei Med J. 1997; 38:359–364.

35. Sohn S, Lee ES, Bang D, Lee S. Behçet's disease-like symptoms induced by the Herpes simplex virus in ICR mice. Eur J Dermatol. 1998; 8:21–23.
36. Sohn S, Lee ES, Kwon HJ, Lee SI, Bang D, Lee S. Expression of Th2 cytokines decreases the development of and improves Behçet's disease-like symptoms induced by herpes simplex virus in mice. J Infect Dis. 2001; 183:1180–1186.

37. Galeone M, Colucci R, D'Erme AM, Moretti S, Lotti T. Potential Infectious Etiology of Behçet's Disease. Patholog Res Int. 2012; 2012:595380.

38. Magro F, Santos-Antunes J, Albuquerque A, Vilas-Boas F, Macedo GN, Nazareth N, et al. Epstein-Barr virus in inflammatory bowel disease-correlation with different therapeutic regimens. Inflamm Bowel Dis. 2013; 19:1710–1716.

39. Dimitroulia E, Pitiriga VC, Piperaki ET, Spanakis NE, Tsakris A. Inflammatory bowel disease exacerbation associated with Epstein-Barr virus infection. Dis Colon Rectum. 2013; 56:322–327.

40. Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999; 94:1582–1586.

41. Goodman AL, Murray CD, Watkins J, Griffiths PD, Webster DP. CMV in the gut: a critical review of CMV detection in the immunocompetent host with colitis. Eur J Clin Microbiol Infect Dis. 2015; 34:13–18.

42. Iida T, Ikeya K, Watanabe F, Abe J, Maruyama Y, Ohata A, et al. Looking for endoscopic features of cytomegalovirus colitis: a study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm Bowel Dis. 2013; 19:1156–1163.
43. Lehner T, Lavery E, Smith R, van der Zee R, Mizushima Y, Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun. 1991; 59:1434–1441.

44. Hirohata S, Oka H, Mizushima Y. Streptococcal-related antigens stimulate production of IL6 and interferon-gamma by T cells from patients with Behcet's disease. Cell Immunol. 1992; 140:410–419.

45. Mendoza-Pinto C, García-Carrasco M, Jiménez-Hernández M, Jiménez-Hernández C, Riebeling-Navarro C, Nava Zavala A, et al. Etiopathogenesis of Behcet's disease. Autoimmun Rev. 2010; 9:241–245.

46. Hasan A, Fortune F, Wilson A, Warr K, Shinnick T, Mizushima Y, et al. Role of gamma delta T cells in pathogenesis and diagnosis of Behcet's disease. Lancet. 1996; 347:789–794.
47. Sartor RB, Mazmanian SK. Intestinal Microbes in Inflammatory Bowel Diseases. Am J Gastroenterol Suppl. 2012; 1:15–21.

48. Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002; 122:44–54.

49. Hansen J, Gulati A, Sartor RB. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol. 2010; 26:564–571.

50. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007; 104:13780–13785.

51. Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004; 53:685–693.

52. Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004; 364:1039–1044.

53. Feller M, Huwiler K, Stephan R, Altpeter E, Shang A, Furrer H, et al. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007; 7:607–613.

54. Abubakar I, Myhill D, Aliyu SH, Hunter PR. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn's disease using nucleic acid-based techniques: a systematic review and meta-analysis. Inflamm Bowel Dis. 2008; 14:401–410.

55. Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J. 2007; 1:403–418.

56. Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007; 5:345–351.

57. Rabizadeh S, Rhee KJ, Wu S, Huso D, Gan CM, Golub JE, et al. Enterotoxigenic bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007; 13:1475–1483.

58. Choi IJ, Kim JS, Cha SD, Jung HC, Park JG, Song IS, et al. Long-term clinical course and prognostic factors in intestinal Behçet's disease. Dis Colon Rectum. 2000; 43:692–700.

59. Lee CR, Kim WH, Cho YS, Kim MH, Kim JH, Park IS, et al. Colonoscopic findings in intestinal Behçet's disease. Inflamm Bowel Dis. 2001; 7:243–249.

60. Cheon JH, Kim WH. An update on the diagnosis, treatment, and prognosis of intestinal Behçet's disease. Curr Opin Rheumatol. 2015; 27:24–31.

61. Jung HC, Rhee PL, Song IS, Choi KW, Kim CY. Temporal changes in the clinical type or diagnosis of Behçet's colitis in patients with aphthoid or punched-out colonic ulcerations. J Korean Med Sci. 1991; 6:313–318.

62. Grigg EL, Kane S, Katz S. Mimicry and deception in inflammatory bowel disease and intestinal Behçet disease. Gastroenterol Hepatol (N Y). 2012; 8:103–112.
63. Zou J, Shen Y, Ji DN, Zheng SB, Guan JL. Endoscopic findings of gastrointestinal involvement in Chinese patients with Behcet's disease. World J Gastroenterol. 2014; 20:17171–17178.

64. Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet's disease and Crohn's disease by colonoscopic findings. Endoscopy. 2009; 41:9–16.

65. Cheon JH, Kim ES, Shin SJ, Kim TI, Lee KM, Kim SW, et al. Development and validation of novel diagnostic criteria for intestinal Behçet's disease in Korean patients with ileocolonic ulcers. Am J Gastroenterol. 2009; 104:2492–2499.

66. Kim JS, Lim SH, Choi IJ, Moon H, Jung HC, Song IS, et al. Prediction of the clinical course of Behçet's colitis according to macroscopic classification by colonoscopy. Endoscopy. 2000; 32:635–640.

67. Chung MJ, Cheon JH, Kim SU, Park JJ, Kim TI, Kim NK, et al. Response rates to medical treatments and long-term clinical outcomes of nonsurgical patients with intestinal Behçet disease. J Clin Gastroenterol. 2010; 44:e116–e122.

68. Yim SM, Kim DH, Lee HJ, Jang HW, Park SJ, Hong SP, et al. Mucosal healing predicts the long-term prognosis of intestinal Behçet's disease. Dig Dis Sci. 2014; 59:2529–2535.

69. Kim DH, Chan HC, Lung PFC, Ng SC, Cheon JH. Ileocolonoscopy in Crohn's disease. In : Kim WH, Cheon JH, editors. Atlas of inflammatory bowel diseases. 1st ed. New York: Springer Berlin Heidelberg;2015. p. 31–51.
70. Shepherd NA. Pathological mimics of chronic inflammatory bowel disease. J Clin Pathol. 1991; 44:726–733.

72. Shen B. Endoscopic, Imaging, and Histologic Evaluation of Crohn's Disease and Ulcerative Colitis. Am J Gastroenterol. 2007; 102:S41–S45.

73. Yurdakul S, Tüzüner N, Yurdakul I, Hamuryudan V, Yazici H. Gastrointestinal involvement in Behçet's syndrome: a controlled study. Ann Rheum Dis. 1996; 55:208–210.
74. Chung JW, Cheon JH, Park JJ, Jung ES, Choi EH, Kim H. Development and validation of a novel prognostic scoring model for ischemic colitis. Dis Colon Rectum. 2010; 53:1287–1294.

75. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976; 70:439–444.
76. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007; 133:423–432.

78. Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991; 12:439–447.

79. van Hees PA, van Elteren PH, van Lier HJ, van Tongeren JH. An index of inflammatory activity in patients with Crohn's disease. Gut. 1980; 21:279–286.

80. Lee HJ, Kim YN, Jang HW, Jeon HH, Jung ES, Park SJ, et al. Correlations between endoscopic and clinical disease activity indices in intestinal Behcet's disease. World J Gastroenterol. 2012; 18:5771–5778.

81. Fresko I, Ugurlu S, Ozbakir F, Celik A, Yurdakul S, Hamuryudan V, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Behçet's syndrome. Clin Exp Rheumatol. 2005; 23:4 Suppl 38. S67–S70.
82. Choi CH, Kim TI, Kim BC, Shin SJ, Lee SK, Kim WH, et al. Anti-Saccharomyces cerevisiae antibody in intestinal Behçet's disease patients: relation to clinical course. Dis Colon Rectum. 2006; 49:1849–1859.

83. Filik L, Biyikoglu I. Differentiation of Behcet's disease from inflammatory bowel diseases: anti-Saccharomyces cerevisiae antibody and anti-neutrophilic cytoplasmic antibody. World J Gastroenterol. 2008; 14:7271.

84. Zeng XJ, Zhu WG, Deng XX, Tang FL, Dong Y. [Anti-endothelial cell antibodies in systemic vasculitis: detection and correlation with disease activity]. Zhonghua Yi Xue Za Zhi. 2004; 84:1629–1632.
85. Lee KH, Chung HS, Kim HS, Oh SH, Ha MK, Baik JH, et al. Human alpha-enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behçet's disease. Arthritis Rheum. 2003; 48:2025–2035.

86. Shin SJ, Kim BC, Kim TI, Lee SK, Lee KH, Kim WH. Anti-alpha-enolase antibody as a serologic marker and its correlation with disease severity in intestinal Behçet's disease. Dig Dis Sci. 2011; 56:812–818.

87. Jung YS, Kim SW, Yoon JY, Lee JH, Jeon SM, Hong SP, et al. Expression of a soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) correlates with clinical disease activity in intestinal Behcet's disease. Inflamm Bowel Dis. 2011; 17:2130–2137.

88. Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, et al. Management of Behçet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behçet disease. Ann Rheum Dis. 2009; 68:1528–1534.

89. Lee HW, Kim WH, Cheon JH. The medical treatments of intestinal Behcet's disease: an update. Intest Res. 2013; 11:155–160.

90. Sawyer A, Walker TM, Terry SI. Behçet's syndrome with ileal involvement--the beneficial effect of sulphasalazine. West Indian Med J. 1978; 27:218–221.
91. Kitauchi S, Ohata H, Kuroda R, Hirose M, Sakaguchi A, Nishi S, et al. [Follow-up observation of intestinal Behçet disease treated with salazosulfapyridine and mesalazine for 8 years and 9 months]. Nihon Shokakibyo Gakkai Zasshi. 1998; 95:140–144.
92. Matsukawa M, Yamasaki T, Kouda T, Kurihara M. Endoscopic therapy with absolute ethanol for postoperative recurrent ulcers in intestinal Behçet's disease, and simple ulcers. J Gastroenterol. 2001; 36:255–258.

93. Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with intestinal Behcet disease. J Clin Gastroenterol. 2012; 46:e38–e45.

95. Kobayashi K, Ueno F, Bito S, Iwao Y, Fukushima T, Hiwatashi N, et al. Development of consensus statements for the diagnosis and management of intestinal Behçet's disease using a modified Delphi approach. J Gastroenterol. 2007; 42:737–745.

96. Park JJ, Kim WH, Cheon JH. Outcome predictors for intestinal Behçet's disease. Yonsei Med J. 2013; 54:1084–1090.

97. Kim DH, Cheon JH, Park JJ, Yoon JY, Moon CM, Hong SP, et al. Clinical outcomes and predictive factors for response after the first course of corticosteroid therapy in patients with Crohn's disease. Gut Liver. 2013; 7:58–65.

98. Yazici H, Pazarli H, Barnes CG, Tüzün Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behçet's syndrome. N Engl J Med. 1990; 322:281–285.

99. Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2012; 18:750–757.

100. Park JJ, Cheon JH, Hong SP, Kim TI, Kim WH. Outcome predictors for thiopurine maintenance therapy in patients with Crohn's disease. Dig Dis Sci. 2012; 57:133–141.

101. Hamuryudan V, Mat C, Saip S, Ozyazgan Y, Siva A, Yurdakul S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behçet syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998; 128:443–450.

102. Yasui K, Uchida N, Akazawa Y, Nakamura S, Minami I, Amano Y, et al. Thalidomide for treatment of intestinal involvement of juvenile-onset Behçet disease. Inflamm Bowel Dis. 2008; 14:396–400.

103. Bariol C, Meagher AP, Vickers CR, Byrnes DJ, Edwards PD, Hing M, et al. Early studies on the safety and efficacy of thalidomide for symptomatic inflammatory bowel disease. J Gastroenterol Hepatol. 2002; 17:135–139.

104. Travis SP, Czajkowski M, McGovern DP, Watson RG, Bell AL. Treatment of intestinal Behçet's syndrome with chimeric tumour necrosis factor alpha antibody. Gut. 2001; 49:725–728.

105. Hassard PV, Binder SW, Nelson V, Vasiliauskas EA. Anti-tumor necrosis factor monoclonal antibody therapy for gastrointestinal Behçet's disease: a case report. Gastroenterology. 2001; 120:995–999.

106. Kram MT, May LD, Goodman S, Molinas S. Behçet's ileocolitis: successful treatment with tumor necrosis factor-alpha antibody (infliximab) therapy: report of a case. Dis Colon Rectum. 2003; 46:118–121.

107. Byeon JS, Choi EK, Heo NY, Hong SC, Myung SJ, Yang SK, et al. Antitumor necrosis factor-alpha therapy for early postoperative recurrence of gastrointestinal Behçet's disease: report of a case. Dis Colon Rectum. 2007; 50:672–676.

108. Lee JH, Kim TN, Choi ST, Jang BI, Shin KC, Lee SB, et al. Remission of intestinal Behçet's disease treated with anti-tumor necrosis factor alpha monoclonal antibody (Infliximab). Korean J Intern Med. 2007; 22:24–27.

109. Naganuma M, Sakuraba A, Hisamatsu T, Ochiai H, Hasegawa H, Ogata H, et al. Efficacy of infliximab for induction and maintenance of remission in intestinal Behçet's disease. Inflamm Bowel Dis. 2008; 14:1259–1264.

110. Iwata S, Saito K, Yamaoka K, Tsujimura S, Nawata M, Suzuki K, et al. Effects of anti-TNF-alpha antibody infliximab in refractory entero-Behcet's disease. Rheumatology (Oxford). 2009; 48:1012–1013.

111. Kinoshita H, Kunisaki R, Yamamoto H, Matsuda R, Sasaki T, Kimura H, et al. Efficacy of infliximab in patients with intestinal Behçet's disease refractory to conventional medication. Intern Med. 2013; 52:1855–1862.

112. Lee JH, Cheon JH, Jeon SW, Ye BD, Yang SK, Kim YH, et al. Efficacy of infliximab in intestinal Behçet's disease: a Korean multicenter retrospective study. Inflamm Bowel Dis. 2013; 19:1833–1838.
113. Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002; 359:1541–1549.

114. Rutgeerts P, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004; 126:402–413.

115. Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004; 350:876–885.

116. De Cassan C, De Vroey B, Dussault C, Hachulla E, Buche S, Colombel JF. Successful treatment with adalimumab in a familial case of gastrointestinal Behcet's disease. J Crohns Colitis. 2011; 5:364–368.

117. Ariyachaipanich A, Berkelhammer C, Nicola H. Intestinal Behçet's disease: maintenance of remission with adalimumab monotherapy. Inflamm Bowel Dis. 2009; 15:1769–1771.
118. van Laar JA, Missotten T, van Daele PL, Jamnitski A, Baarsma GS, van Hagen PM. Adalimumab: a new modality for Behçet's disease? Ann Rheum Dis. 2007; 66:565–566.
119. Tanida S, Inoue N, Kobayashi K, Naganuma M, Hirai F, Iizuka B, et al. Adalimumab for the treatment of Japanese patients with intestinal Behçet's disease. Clin Gastroenterol Hepatol. 2015; 13:940–948.

120. Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006; 130:323–333.

121. Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007; 56:1232–1239.

122. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007; 132:52–65.

123. Hisamatsu T, Ueno F, Matsumoto T, Kobayashi K, Koganei K, Kunisaki R, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet's disease: indication of anti-TNFα monoclonal antibodies. J Gastroenterol. 2014; 49:156–162.

124. Jung YS, Yoon JY, Lee JH, Jeon SM, Hong SP, Kim TI, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2011; 17:1594–1602.

125. Lee KS, Kim SJ, Lee BC, Yoon DS, Lee WJ, Chi HS. Surgical treatment of intestinal Behçet's disease. Yonsei Med J. 1997; 38:455–460.

126. Moon CM, Cheon JH, Shin JK, Jeon SM, Bok HJ, Lee JH, et al. Prediction of free bowel perforation in patients with intestinal Behçet's disease using clinical and colonoscopic findings. Dig Dis Sci. 2010; 55:2904–2911.

127. Naganuma M, Iwao Y, Inoue N, Hisamatsu T, Imaeda H, Ishii H, et al. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behçet's disease. Am J Gastroenterol. 2000; 95:2848–2851.

128. Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990; 211:622–627.
129. Larson DW, Pemberton JH. Current concepts and controversies in surgery for IBD. Gastroenterology. 2004; 126:1611–1619.

130. Sayek I, Aran O, Uzunalimoglu B, Hersek E. Intestinal Behçet's disease: surgical experience in seven cases. Hepatogastroenterology. 1991; 38:81–83.
131. Chou SJ, Chen VT, Jan HC, Lou MA, Liu YM. Intestinal perforations in Behçet's disease. J Gastrointest Surg. 2007; 11:508–514.

132. Iida M, Kobayashi H, Matsumoto T, Okada M, Fuchigami T, Yao T, et al. Postoperative recurrence in patients with intestinal Behçet's disease. Dis Colon Rectum. 1994; 37:16–21.

133. Matsumoto T, Uekusa T, Fukuda Y. Vasculo-Behçet's disease: a pathologic study of eight cases. Hum Pathol. 1991; 22:45–51.

134. Bozkurt M, Torin G, Aksakal B, Ataoglu O. Behçet's disease and surgical intervention. Int J Dermatol. 1992; 31:571–573.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download