Abstract
Purpose
Smoking reportedly exerts deleterious effects on renal function; however, its effects on histology have not been clarified in patients with IgA nephropathy (IgAN).
Materials and Methods
Renal histology was evaluated in a cohort of 397 patients diagnosed with IgAN according to smoking status and dose in relation to renal function.
Results
Among the study cohort, which was predominantly male (88.5%), 52 patients (13%) were current smokers. These current smokers demonstrated more frequent hypertension and higher serum creatinine levels than non/ex-smokers at the time of diagnosis, which was apparent with increased smoking dose. The percentages of global glomerulosclerosis and arteriolar hyalinosis increased with increased smoking dose, whereas tubulointerstitial fibrosis or arterial intimal thickening did not. Glomerular mesangial alpha-smooth muscle actin expression were similar between current and non/ex-smokers matched for age, gender, hypertension, and histologic severity, although the number of glomerular CD68+ cells was significantly fewer in smokers. Initial serum creatinine level, estimated glomerular filtration rate (eGFR), and global glomerulosclerosis were found to be risk factors of serum creatinine doubling in both smokers and non/ex-smokers by univariate analysis during a mean follow-up of 3.8 years.
IgA nephropathy (IgAN) is one of the most common primary glomerulonephritis worldwide. It slowly progresses to end stage renal disease (ESRD) within 20 years of onset in about one third of patients. Proteinuria, hypertension, and elevated serum creatinine at presentation are the best known clinical factors related to disease progression.1 However, clinical courses vary greatly from patient to patient; metabolic, environmental, and uncertain genetic factors may have some role in this.23
Smoking is a well-known risk factor of cardiovascular disease, as well as renal dysfunction.45 67 In IgAN patients, cigarette smoking was reported to elevate serum creatinine levels and to be associated with renal disease progression.89 However, research has yet to clarify whether cigarette smoking exerts its effects by triggering hemodynamic derangement or by aggravation of renal structural changes. Circumstantial evidence and experimental data support the involvement of histologic damage. Arterio- and arteriolosclerosis was reported to be a main histologic feature related to smoking.10 In addition, the glomerular mesangium, which is activated by aberrantly glycosylated IgA11112 may receive additional oxidative stress elicited by cigarette smoke in IgAN patients.13 In animal experiments, administration of nicotine, a major component of cigarette smoke, caused mesangial proliferation and matrix increase.14 Under the assumption of renal histologic progression by smoking, a retrospective assessment of renal histology was performed according to smoking history and dose. We further performed glomerular immunohistology and arterial morphometry to reveal the major sites of histologic injury upon smoking. Additionally, the influence of smoking on risk factors predictive of renal functional decline was explored.
A total of 397 IgAN cases diagnosed at the Department of Pathology between January 2005 and December 2012 were retrieved from the clinical data repository of Yonsei University Severance Hospital. The inclusion criteria were as follows: age over 18 years at time of diagnosis; documentation of smoking history in electronic medical charts; and histologic confirmation of IgAN by predominant mesangial IgA deposition on immunofluorescent microscopy and at least six glomeruli on light microscopy. We excluded cases with diabetes mellitus, hepatitis B or C viral carriers, renal transplantation patients or other renal disease patients, except for those with IgAN and hypertension. Smokers were further divided into three subgroups according to smoking amounts: <5 pack-years (PY), 5-15 PY, and >15 PY.8 Renal functional decline after biopsy was assessed in cases in which urinary protein excretion and serum creatinine were measured more than two times at a 6-month interval and follow-up times exceeded one year. The design and methods of the study were approved by the Institutional Review of Board of our institution.
Renal biopsy samples were processed for light microscopy, immunofluorescence, and electron microscopy. For light microscopy, formalin fixed, paraffin embedded sections were cut to 3 um and stained with hematoxylin-eosin, periodic acid-Schiff (PAS), aldehyde fuchsin orange G, and periodic acid-silver methenamine methods. IgAN was diagnosed as the presence of predominant mesangial IgA deposition on immunofluorescence. The overall histologic severity was measured by the Haas subclass.15 The percentages of global and segmental glomerulosclerosis were calculated in each case. The degrees of tubular atrophy and interstitial fibrosis were graded from 0 to 3 (0, negative; 1, less than 25% of cortex affected; 2, 25-50% of cortex affected; and 3, >50% of cortex affected) and were grouped into none to mild (0-1) and moderate to severe (2-3) subgroups for statistical analysis of follow-up data. All arterioles in PAS-stained slides were evaluated for the presence of arteriolar hyalinosis. In arteries, the presence of arterial intimal thickening and its quantity were assessed using the image J program. Every available artery in PAS-stained slides was captured under ×200 magnification. The whole thickness of thickened and normal areas was measured in each artery and the ratio was calculated. Histologic and morphometric evaluation was made without access to clinical information.
Thirty-nine cases each among smokers and non-smokers were selected by 1:1 matching for age, gender, hypertension, and IgAN subclass, applying propensity score matching method using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA). Deparaffinized, 3-um thick renal sections were incubated with primary antibody against alpha-smooth muscle actin (α-SMA) (1A4, 1:1000, Dako, Glostrup, Denmark) and CD68 (PG-M1, 1:500, Dako), and visualized using a DAB Map kit on Discovery XT (Ventana Medical Systems, Tucson, AZ, USA). Sections omitting primary antibody were used as negative controls. Glomerular α-SMA expression was evaluated only in nonsclerotic glomeruli demonstrating vascular hilum or a urinary pole. With image J, α-SMA stained areas were measured and the percentage thereof was calculated by dividing the sum of α-SMA area by the average glomerular area. Intraglomerular CD68+ macrophages were counted in nonsclerotic glomeruli and expressed as the average number per glomerulus.
Age, gender, hypertension, smoking and its amount, duration of follow-up, serum creatinine, serum IgA levels, and proteinuria at the time of diagnosis and last follow-up were retrieved from electronic medical charts. Urinary protein and creatinine concentrations were measured from a random spot urine sample, and a urinary protein/creatinine ratio (UPCR) (g/g) was calculated as an index of proteinuria. Time-average (TA) proteinuria was determined by TA-UPCR, averaging UPCRs obtained in 6-month intervals after biopsy.16 Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula. Renal outcome was measured by doubling of serum creatinine above baseline serum creatinine level.
Results were expressed as mean±standard deviation. Data were analyzed with SPSS software for Windows (version 18.0; SPSS Inc., Chicago, IL, USA). Mann-Whitney U test was used to compare continuous variables, and Pearson's chi-square test and Fisher's exact test were used to compare categorical variables. Wilcoxon signed rank test was used for comparison of matched patients. Kruskal-Wallis test was used to compare differences among the three smoker subgroups divided according to smoking dose. A repeated measure ANOVA was used to assess changes in eGFR, serum creatinine, and UPCR. A Cox proportional hazards model was used to assess risk factors for serum creatinine doubling in follow-up cases. p<0.05 was considered significant.
In a cohort of 397 IgAN cases, 52 patients were current smokers and 345 patients were non/ex-smokers. The current smokers were predominantly male. Mean age was similar in both groups, but significantly increased among smokers as smoking dose increased. Hypertension was more frequent and serum creatinine level was significantly higher in current smokers than in non/ex-smokers at the time of diagnosis; both increased markedly with higher smoking doses. UPCR and eGFR were not significantly different between smokers and non/ex-smokers, but significantly increased in heavy smokers in comparison to light smokers. Serum IgA quantitation was available in 305 patients at the time of biopsy. Mean serum IgA levels were not statistically different between smokers and non/ex-smokers. Heavy smokers tended to have higher IgA levels than those of light smokers, although the levels were not statistically significant (Table 1 and 2).
When renal histology was compared between smokers and non/ex-smokers, neither Haas histologic grades nor histological parameters were significantly different (Table 3). The prevalence of arterial intimal thickening or the ratio of vascular wall thickness was not different according to smoking status or dose. Meanwhile, the percentages of global glomerulosclerosis and arteriolar hyalinosis were significantly increased as smoking dose increased in current smokers (Table 4).
Glomerular injury by myofibroblastic activation and/or inflammatory cells was assessed by quantitation of α-SMA+ mesangial areas and intraglomerular CD68+ cells after matching age, gender, and histologic severity in current and non/ex-smokers. α-SMA+ glomerular mesangial areas were similar between current and non/ex-smokers; however, the number of CD68+ cells was significantly less in smokers (Table 5). CD68+ cell infiltration did not show correlation with any clinical or histologic variables or α-SMA expression by univariate analysis. In multivariate analysis, however, global glomerulosclerosis was negatively correlated and segmental glomerulosclerosis was positively correlated with CD68+ cell infiltration (Table 6).
Data for use in evaluating renal functional decline was available in 283 IgAN patients. After a mean follow-up of 3.8 years, serum creatinine levels were significantly higher and eGFR tended to be lower in current smokers than in non/ex-smokers. However, the proportion of patients with serum creatinine level elevated 50% or more from baseline (Δ serum creatinine ≥50%) at last follow-up was not different between smokers and non/ex-smokers. Proteinuria measured as UPCR was significantly increased in current smokers and decreased in non/ex-smokers after follow-up (Table 7). Serum IgA levels were measured more than twice in 71 patients during follow-up: IgA levels tended to decrease in both groups. However, the rate of decrease was not significantly different between current smokers (-0.01±0.16, n=7) and non/ex-smokers (-0.33±0.16, n=64) (p=0.732).
When risk factors of serum creatinine doubling were assessed in all IgAN patients, hypertension, serum creatinine level, eGFR, global glomerulosclerosis, tubular atrophy, and arteriolar hyalinosis were significant in univariate Cox proportional hazards analysis. However, when the cases were divided into smokers and non/ex-smokers, the risk factors included serum creatinine level, eGFR, and global glomerulosclerosis in both groups by univariate analysis and eGFR in non/exsmokers by multivariate analysis (Table 8).
In addition to intrinsic immune complex-mediated renal injury, environmental stimuli and personal habits may further trigger disease activity and progression in patients with IgAN. In the present study, we explored smoking effects on renal function and histology in IgAN patients. Smoking showed deleterious effects on renal function. Serum creatinine levels were higher in smokers than in non/ex-smokers. With increasing smoking dose, the prevalence of hypertension, serum creatinine level, and urinary protein excretion increased significantly. Current smokers were predominantly male and of older age as smoking dose increased. Interestingly, male gender and older age were reported to be risk factors for ESRD in some studies of IgAN,1718 which raises suspicions of smoking being an offender. Moreover, estrogen deficiency may increase nicotine-induced renal injury.19
Renal histology in terms of Haas grades or individual histologic parameters was not different between current smokers and non/ex-smokers. Haas grades tended to be higher in heavy smokers than in light smokers, although without statistical significance. We further tested whether membranoproliferative features are associated with smoking. Five cases (one current smoker and four non/ex-smokers) showed a double contour feature of glomerular capillary walls, and four of them had subendothelial electron dense deposits. However, statistical analysis was not possible due to the small number of patients having these features. In contrast, the percentage of global glomerulosclerosis and the prevalence of arteriolar hyalinosis increased with increased smoking doses. While it would be difficult for us to judge which of the two lesions comes first, the presence of arteriolar hyalinosis may hamper glomerular perfusion and increase the speed of glomerulosclerosis. In contrast to microvascular changes, the degrees of tubulointerstitial fibrosis and arteriosclerosis were not different according to smoking status or doses. Tubulointerstitial fibrosis was generally mild: 11.5% of smokers and 7% of non/ex-smokers showed moderate to severe interstitial fibrosis. In heavy smokers, its prevalence increased to 16.7%. As we used a crude fourtiered scale in the assessment of interstitial fibrosis, quantitative morphometric assessment might have revealed subtle differences. Since neither the presence of arteriosclerosis nor arterial wall thickness differed between smokers and non/exsmokers, we thought that glomeruli and arterioles are the main targets of smoking-related injury in IgAN patients. These findings are different from those reported by Lhotta, et al.,10 who claimed small interlobular arteries to be the main targets of smoking-related injury. This discrepancy may be attributed to differences in smoker grouping and the study cohorts between the two studies. We divided cases into current smokers and non/ex-smokers at the time of biopsy, instead of neversmokers and ever-smokers. More importantly, we comprised only IgAN cases of which histologic severity of each compartment was assessed using the same evaluation method, whereas their study included various glomerular diseases with diverse etiology, clinical presentation, and histology,10 and thus, only vascular assessment was feasible.
As ancillary markers of glomerular injury, glomerular CD68+ macrophage infiltration and mesangial α-SMA expression were compared between smokers and non/ex-smokers. Glomerular α-SMA expression is regarded as myofibroblastic transformation of mesangial cells and a marker of mesangial cell activation.20 It has been reported to be related to disease activity21 and renal functional decline in IgAN patients.22 Nicotine treatment has been found to induce mesangial cell proliferation and fibronectin production in human mesangial cell culture.14 However, in the present study, the extent of mesangial α-SMA+ areas did not differ according to smoking status, rather by histologic severity based on Haas grades. As cases were previously matched for Haas grades, we thought that similar histologic injuries could not be further distinguished by α-SMA expression. On the contrary, the number of CD68+ cells was significantly less in smokers than in non/ex-smokers. CD68+ cells showed no significant correlation with any clinical or histologic factors or α-SMA expression in univariate analysis, although global glomerulosclerosis was negatively correlated and segmental glomerulosclerosis was positively correlated with CD68+ cell infiltration in multivariate analysis. Glomerular macrophage infiltration was previously reported to be correlated with mesangial and endocapillary proliferation in children23 and with glomerular matrix expansion and segmental/global glomerulosclerosis in adults with IgAN.24 In a proteinuric rat model, long-term oral nicotine-treatment reduced glomerular macrophage infiltration, glomerulosclerosis, and interstitial α-SMA expression.25 Our results led us to speculate that macrophages are associated with an active state of glomerular matrix laydown and sclerosis. As macrophages play different roles in inflammation and tissue repair depending on subsets of patients,2426 further clarification of whether macrophage phenotypes differ according to smoking status and stages of IgAN is needed.
In the present study, the risk factors of serum creatinine doubling were not different according to smoking status. Renal function at time of diagnosis and global glomerulosclerosis were significant in both smokers and non/ex-smokers in univariate analysis. The speed of renal functional decline was similar in both groups, as shown by the proportion of patients with Δ serum creatinine ≥50% at last follow-up. Proteinuria per se was not significant either. However, increased proteinuria after follow-up may suggest disease progression in smokers.23 Taken together, the lack of significant harmful effects on renal disease progression by smoking seems to implicate a dominant role of functional derangement over irreversible histological changes by smoking. Nevertheless, the short follow-up period, the small proportion of smokers, and the only 3.5% of cases with creatinine doubling may prevent us from unveiling definite histologic clues.
The study has several limitations. First, it was not certain whether smoking status at diagnosis would be continued during follow-up in our patients. However, we expected that the number of patients who changed smoking habits might be negligible over the 3.8 year follow-up period. Among clinical factors, age was significantly increased with increased smoking dose. Although we could not completely eliminate age effects on renal histology, significant changes were limited to glomeruli and arterioles in old heavy smokers. When we evaluated the influence of aging on histology in non/ex-smokers, significant progression occurred in all histologic compartments by univariate analysis and in interstitium, arterioles, and arteries by multivariate analysis by linear regression analysis (data not shown). Another factor omitted in the evaluation was effects of angiotensin converting enzyme inhibitors (ACEI) on IgAN and smoking-induced renal dysfunction.27 Although we did not check the proportion of patients under ACEI treatment, TA proteinuria was not different between the two groups. As we mentioned previously, the relatively short follow-up might have masked more definite evidence, although the mixed effects of glomerulonephritis, smoking, and others might be an obstacle for interpretation in advanced stage disease.
In conclusion, smoking causes dose dependent harmful effects on the kidneys in IgAN patients. In addition to renal functional decline and elevated blood pressure, glomerulosclerosis and arteriolar hyalinosis may contribute to renal disease progression in these patients.
Figures and Tables
Table 1
Patient's Demography According to Smoking Status at Time of Diagnosis (n=397)

Table 2
Clinical Parameters According to Smoking Dose at Time of Diagnosis

Table 3
Renal Histology According to Smoking Status
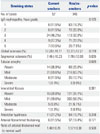
Table 4
Renal Histology According to Smoking Dose
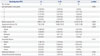
Table 5
Clinical Features and Renal Histology in a 1:1 Matched Cohort of Current and Non- or Ex-Smokers in IgAN Patients (Total n=78)

Table 6
Regression Analysis for Glomerular CD68 Infiltration in a 1:1 Matched Cohort

Table 7
Clinical Course According to Smoking Status and Dose

Table 8
Risk Factors of Serum Creatinine Doubling by a Cox Proportional Hazards Model

References
1. D'Amico G, Imbasciati E, Barbiano Di Belgioioso G, Bertoli S, Fogazzi G, Ferrario F, et al. Idiopathic IgA mesangial nephropathy Clinical and histological study of 374 patients. Medicine (Baltimore). 1985; 64:49–60.
2. D'Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2004; 24:179–196.
3. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76:546–556.

4. Halimi JM, Giraudeau B, Vol S, Cacès E, Nivet H, Lebranchu Y, et al. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int. 2000; 58:1285–1292.

5. Stengel B, Couchoud C, Cénée S, Hémon D. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies. Kidney Int. 2000; 57:2519–2526.

6. Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, et al. Smoking is associated with renal impairment and proteinuria in the normal population: the AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis. 2002; 40:704–712.

7. Ejerblad E, Fored CM, Lindblad P, Fryzek J, Dickman PW, Elinder CG, et al. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol. 2004; 15:2178–2185.

8. Orth SR, Stöckmann A, Conradt C, Ritz E, Ferro M, Kreusser W, et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int. 1998; 54:926–931.

9. Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, et al. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis. 2010; 56:313–324.

10. Lhotta K, Rumpelt HJ, König P, Mayer G, Kronenberg F. Cigarette smoking and vascular pathology in renal biopsies. Kidney Int. 2002; 61:648–654.

11. Coppo R, Camilla R, Amore A, Peruzzi L. Oxidative stress in IgA nephropathy. Nephron Clin Pract. 2010; 116:c196–c198.

12. Camilla R, Suzuki H, Daprà V, Loiacono E, Peruzzi L, Amore A, et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011; 6:1903–1911.

13. Raza H, John A, Nemmar A. Short-term effects of nose-only cigarette smoke exposure on glutathione redox homeostasis, cytochrome P450 1A1/2 and respiratory enzyme activities in mice tissues. Cell Physiol Biochem. 2013; 31:683–692.

14. Jaimes EA, Tian RX, Joshi MS, Raij L. Nicotine augments glomerular injury in a rat model of acute nephritis. Am J Nephrol. 2009; 29:319–326.

15. Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997; 29:829–842.

16. Reich HN, Troyanov S, Scholey JW, Cattran DC. Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007; 18:3177–3183.

17. Nicholls KM, Fairley KF, Dowling JP, Kincaid-Smith P. The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 1984; 53:227–250.
18. Packham DK, Yan HD, Hewitson TD, Nicholls KM, Fairley KF, Kincaid-Smith P, et al. The significance of focal and segmental hyalinosis and sclerosis (FSHS) and nephrotic range proteinuria in IgA nephropathy. Clin Nephrol. 1996; 46:225–229.
19. Elliot SJ, Karl M, Berho M, Xia X, Pereria-Simon S, Espinosa-Heidmann D, et al. Smoking induces glomerulosclerosis in aging estrogen-deficient mice through cross-talk between TGF-beta1 and IGF-I signaling pathways. J Am Soc Nephrol. 2006; 17:3315–3324.

20. Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992; 41:1134–1142.

21. Kaneko Y, Nakazawa K, Higuchi M, Hora K, Shigematsu H. Glomerular expression of alpha-smooth muscle actin reflects disease activity of IgA nephropathy. Pathol Int. 2001; 51:833–844.

22. Utsunomiya Y, Kawamura T, Abe A, Imai H, Hirano K, Maruyama N, et al. Significance of mesangial expression of alpha-smooth muscle actin in the progression of IgA nephropathy. Am J Kidney Dis. 1999; 34:902–910.

23. Nagata M, Akioka Y, Tsunoda Y, Komatsu Y, Kawaguchi H, Yamaguchi Y, et al. Macrophages in childhood IgA nephropathy. Kidney Int. 1995; 48:527–535.

24. Ikezumi Y, Suzuki T, Karasawa T, Hasegawa H, Yamada T, Imai N, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology. 2011; 58:198–210.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download