Abstract
An adenomyomectomy is a conservative-surgical option for preserving fertility. Conventional laparoscopic adenomyomectomies present difficulties in adenomyoma removal and suturing of the remaining myometrium. Robot-assisted laparoscopic surgery could overcome the limitations of conventional laparoscopic surgery. Four patients with severe secondary dysmenorrhea and pelvic pain visited Seoul St. Mary's Hospital and were diagnosed with adenomyosis by pelvic ultrasonography and pelvic magnetic resonance imaging (MRI). The four patients were unmarried, nulliparous women, who desired a fertility-preserving treatment. We performed robot-assisted laparoscopic adenomyomectomies. The dysmenorrhea and pelvic pain of the patients nearly disappeared after surgery. No residual adenomyosis was observed on the follow-up pelvic MRI. A robot-assisted laparoscopic adenomyomectomy was feasible, and could be a minimally invasive surgical option for fertility-sparing treatment in patients with adenomyosis.
Adenomyosis is a common benign gynecological disease characterized by ectopic endometrial glands and stroma within the myometrium. It typically affects premenopausal women who typically suffer from dysmenorrhea, menorrhagia, chronic pelvic pain, and infertility.1 The standard treatment of adenomyosis is a hysterectomy; treating symptomatic women who want to maintain fertility is challenging.2 Treatment should be individualized. For preserving fertility and relieving symptoms, medical treatment is the first choice. If dysmenorrhea or chronic pelvic pain does not respond to medical treatment or if anemia occurs from menorrhagia, excisional surgery would eventually be inevitable. An adenomyomectomy is a conservative surgical option for treating adenomyosis while preserving fertility; we describe an unreported surgical adenomyomectomy method, known as robot-assisted laparoscopic adenomyomectomy.
Four patients with severe secondary dysmenorrhea and pelvic pain visited Seoul St. Mary's Hospital between October 2012 and July 2013. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital according to the Bioethics and Safety Act and the Declaration of Helsinki (IRB ID-KC14RISI0607). A thorough clinical history including age, marital status, parity, age at menarche, occupation, education, and type and severity of symptoms was obtained. The four patients were unmarried, nulliparous woman, who were college graduates. Their mean age was 33±2.94 years old, and their mean body mass index was 25.3±5.23 (Table 1). Adenomyosis was diagnosed by pelvic ultrasonography. To determine the exact location of and size of the lesion as well as its relationship to the uterine cavity, all the patients underwent pelvic magnetic resonance imaging (MRI). The preoperative and postoperative severity of dysmenorrhea and pelvic pain were recorded according to a visual analog scale (VAS) ranging from 0 (no pain) to 10 (extremely severe pain). The mean VAS during the menstrual period before surgery was 9.0±1.41. Before and after the operation, the serum level of CA-125 was checked in all the patients. Serum CA-125 levels could reflect the activity of adenomyosis.3 The mean serum level of CA-125 before surgery was 104.09±125.38 U/mL. The surgery was performed under general anesthesia. The basic robotic system included the patient-side robot, a vision cart, and the surgeon's console. The patient was placed in the dorsal lithotomy position with the arms and shoulders protected with cotton pads. One 12mm trocar was positioned in the umbilicus for the placement of the camera. Two 8-mm trocars were placed in the right and left lower quadrants for the two robotic arms. One 5-mm trocar was placed in the right upper quadrant for the 1st assistant to perform suction/irrigation and for the passage of the suture materials. Our preferred robotic instruments for this operation include curved monopolar scissors, tenaculum forceps, PK bipolar forceps and mega needle drivers. After the exact location of the adenomyoma was determined by a visual inspection and MRI review, diluted vasopressin (vasopressin 10 IU in 100 mL of normal saline) was injected into the serosa and myometrium surrounding the adenomyoma. Using curved monopolar scissors without electrocauterization (also called a 'cold-cut'), we performed a horizontal incision over the vertex of the adenomyoma. Because the robotic vision system was equipped with a high-definition, three-dimensional (3D) endoscope, we could identify the normal myometrium and the adenomyoma. Using the 'cold-cut' method with curved monopolar scissors and minimal electrocauterization, we excised the adenomyoma. After controlling the bleeding, we repaired the remaining myometrium with a 2-0 Vicryl® suture (Ethicon, Inc., Somerville, NJ, USA). Because the adjacent myometrium is easily lacerated by the suture material, we repaired the myometrium layer-by-layer interruptedly. For meticulous suturing without dead space, we sutured the myometrium to a sufficient depth with enough tissue to prevent laceration. After completion of the myometrial sutures, we repaired the serosal layer with a baseball suture to prevent adhesion formation after surgery (Fig. 1). We inserted a levonorgestrel-releasing intrauterine system in the patients immediately after completion of the operation as an adjuvant treatment of adenomyosis because all the patients were unmarried and did not have a plan for pregnancy in the near future.
The mean operation time was 159.25±93.06 minutes, and the mean hospital stay was 2.5±0.5 days. The mean estimated blood loss during the operation was 117.5±56.78 mL. Dysmenorrhea and pelvic pain nearly disappeared one or two weeks after the surgery. The mean serum CA-125 level 6 months after surgery was 6.17±1.97 U/mL (Table 1). Six months after surgery, each patients underwent a pelvic MRI to evaluate the uterine status. No residual adenomyosis was observed on the pelvic MRI (Fig. 2). All four patients were observed 2.13 years after surgery. All these four patients are doing well without any symptoms.
Adenomyosis is most frequently identified in women in their forties and fifties.4 Because of the recent trend of delayed marriage and childbearing, premenopausal adenomyosis might increasingly become a factor affecting fertility. The patients in this study were young, unmarried, nulliparous women who wanted to maintain fertility. The standard management of adenomyosis has not been established for women who wish to preserve fertility.
In the patients with diffuse adenomyosis, laparotomic adenomyomectomy was the method of choice for radical resection of adenomyomatous tissue and for preservation of fertility. In the patients with focal adenomyosis exclusively, a minimally invasive surgical approach could be possible. Adenomyosis has an unclear demarcation from the normal myometrium; because of this characteristic, conventional laparoscopy has disadvantages in the absence of palpation of the uterus, including inaccurate assessment of the extent of adenomyosis and repair of a myometrial defect in a limited range of motion. Robotic surgery, in contrast to conventional laparoscopy, has a better visual field, with 3D HD vision, and the robotic arm has an Endowrist® (Intuitive Surgical, Inc., Sunnyvale, CA, USA) with seven degrees of freedom that ensures repairing the remaining myometrium layer-by-layer as similar to laparotomic suturing as possible. Compared to a laparotomic adenomyomectomy, robotic surgery has strengths that could facilitate adenomyomectomy as a minimally invasive surgery. Laparotomic surgery results in a greater estimated blood loss and a longer hospital stay and recovery time, and it causes more adhesion formation than minimally invasive surgery.567 The robot-assisted laparoscopic adenomyomectomy method enables suturing as meticulous as in open surgery and results in less estimated blood loss, shorter hospital stays and recovery times than open surgery. Robot-assisted laparoscopic surgery has the advantages of open surgery and minimally invasive surgery. Although we should wait for the pregnancy rate and outcomes of the patients, the robot-assisted laparoscopic adenomyomectomy method is a technically feasible and minimally invasive surgical option for fertility-sparing treatment. The effectiveness and safety of robot-assisted laparoscopic adenomyomectomy have not yet been fully evaluated at this stage, and further study is needed.
Figures and Tables
Fig. 1
Operative procedures. (A and B) Injection of diluted vasopressin into an adenomyoma. (C and D) Removal of an adenomyoma from the uterus with minimal thermal damage. (E) Bleeding control. (F) Repair of the remaining myometrium and serosa.
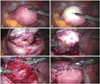
Fig. 2
Preoperative and postoperative MRI findings. (A) Preoperative image. (B) Postoperative image. MRI, magnetic resonance imaging.
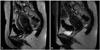
Table 1
Patients' Characteristics and Preoperative and Postoperative Changes in Symptom and CA-125

References
1. Levy G, Dehaene A, Laurent N, Lernout M, Collinet P, Lucot JP, et al. An update on adenomyosis. Diagn Interv Imaging. 2013; 94:3–25.

2. Pepas L, Deguara C, Davis C. Update on the surgical management of adenomyosis. Curr Opin Obstet Gynecol. 2012; 24:259–264.

3. Hirata T, Izumi G, Takamura M, Saito A, Nakazawa A, Harada M, et al. Efficacy of dienogest in the treatment of symptomatic adenomyosis: a pilot study. Gynecol Endocrinol. 2014; 30:726–729.

4. Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011; 18:428–437.

5. Postoperative adhesion development after operative laparoscopy: evaluation at early second-look procedures. Operative Laparoscopy Study Group. Fertil Steril. 1991; 55:700–704.
6. Luciano AA, Maier DB, Koch EI, Nulsen JC, Whitman GF. A comparative study of postoperative adhesions following laser surgery by laparoscopy versus laparotomy in the rabbit model. Obstet Gynecol. 1989; 74:220–224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download