Abstract
The pulmonary interstitial emphysema (PIE) is a life-threatening illness in premature infants with mechanical ventilation. While most are managed conservatively, decompression would be necessary. Here, we report the first case of PIE treated by percutaneous catheter insertion in an extremely low birth weight (ELBW) infant in Korea. The patient, born with 660 g in 23+2 weeks of gestation, showed PIE in left lower lung on postnatal day 12. Percutaneous catheter insertion was performed on postnatal day 25. The size of PIE decreased, but didn't disappear completely. On postnatal day 42, we exchanged catheter and inserted additional catheter in pleural space. However, sudden desaturation and pneumothorax occurred on postnatal day 44. We changed catheter in pleural space, and pneumothorax and PIE improved. Finally, we successfully removed catheters, and weaned patient out. As in our case, percutaneous catheter insertion would be a useful option for ELBW infants with PIE.
The pulmonary interstitial emphysema (PIE) is known as a risk factor of mortality and morbidity related with mechanical ventilation in premature infants with extremely low birth weight (ELBW). By surgical resection, ventilator strategies, steroid administration and selective intubation, PIE can be treated, but percutaneous catheter insertion is also considered as a treatment option for PIE. Herein, we report a case of PIE in an infant with ELBW treated by percutaneous catheter insertion.
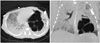
A female of 660 g, at a 23+2 weeks of gestation, was born through cesarean section to 38-year-old, gravida 1, para 0 with acute choriodeciduitis, immature synchronous placenta. Antenatal steroid administration was completed. The Apgar scores of the baby were 1 and 3 at 1 and 5 minutes, respectively. Therefore, positive pressure ventilation was applied immediately after birth. Surfactant replacement therapy with synchronized intermittent mandatory ventilation was also applied to the patient when admitted to neonatal intensive care unit. Surfactant replacement therapy was administered one more time at 57 hours after birth due to relapse of respiratory distress syndrome. High frequency oscillatory ventilation (HFOV) was provided by a Drager Babylog VN500 (Dragerwerk Ag & Co., Lubeck, Germany) for adequate ventilation since postnatal day 3. Steroid was administered for adequate ventilation in short period (hydrocortisone 1 mg/kg on day 9, 0.5 mg/kg twice on day 10). On postnatal day 12, a PIE in the left lower lobe was observed on chest X-ray, and the size of emphysema increased gradually (Fig. 1A). Mean airway pressure used on the HFOV was 7 cm H2O. Frequency was set at 12 Hz and amplitude was 14 cm H2O with 0.6 of FiO2. Despite of HFOV with low oscillatory frequency and intermittent one lung ventilation, the PIE expanded progressively which shifted of the mediastinum to the right side (Fig. 1B). We decided to try percutaneous catheter insertion to evacuate the PIE. Thus, with local anesthesia, a central venous catheter (14 Gauge, Arrow International, Reading, PA, USA) was inserted at left lower side of lung on day 25. After insertion, the size of PIE decreased, but it still remained (Fig. 1C). We tried clamping of catheter drainage on day 30 for catheter removal, but PIE was aggravated (Fig. 1D). On day 42, we changed catheter at the same location for proper positioning, and additional central intravenous catheter was inserted into pleural space to avoid air leakage. Chest computed tomography (CT) taken on the same day showed multilobulat-ed cystic mass, consistent with PIE in left lower lobe (Fig. 2). On day 44, sudden desaturation and respiratory distress occurred due to tension pneumothorax. On day 45 the central venous catheter in pleural space was changed to trocar catheter (10 French, Pacific Hospital Supply, Taipei, Taiwan) with suction pressure of -20 mm H2O for the sufficient evacuation (Fig. 1E and F). As pneumothorax was alleviated, the catheter in the pleural space was removed on day 53. We clamped the catheter inserted in PIE on the same day. There was no in-crease in the size of PIE for 48 hours. After assuring no recurrence, we successfully removed the drainage on day 56 (Fig. 1G). She was weaned from HFOV successfully on day 57 (Fig. 1H), and nasal continuous positive airway pressure was applied on day 59. Moderate grade of chronic lung disease was diagnosed. As co-morbidities, patent ductus arteriosus was closed by oral ibuprofen, and retinopathy of prematurity with stage II was treated by ranibizumab injection. She was discharged on day 109 without any support.
PIE is a life threatening condition of prematurity with the respiratory distress syndrome with mortality reported as high as 53–67%.1 PIE derives from air leakage into interstitium from alveolus due to disruption of the alveolar wall basement membrane.2 About 2% of premature infants in neonatal intensive care unit experience PIE.3 For premature infants with respiratory distress syndrome, the incidence rate goes up to 20–32%.145 In a retrospective case-controlled study, 24% of ELBW infants developed PIE.6
Prematurity, low birth weight, low Apgar score, high peak inspiratory pressure over 25 cm H2O of mechanical ventilation, and in utero exposure to MgSO4 are known risk factors of PIE.78 In our case, the patient was 23+2 weeks of gestation, and 660 g with Apgar score 1 and 3 at 1 and 5 minutes, respectively. Maximal peak inspiratory pressure was 15 cm H2O, and maximal mean airway pressure was 10 cm H2O. At the day of PIE first detected, mean airway pressure used on the HFOV was 7 cm H2O. Also, there was no prenatal exposure to MgSO4. PIE is associated with late development of chronic lung disease in prematurity.9 In our case, the patient was diagnosed as moderate grade of chronic lung disease.
Prenatal steroids and postnatal surfactant replacement independently and additively reduce mortality, the severity of respiratory distress syndrome, and air leaks in preterm infants.10 Antenatal steroid administration (dexamethasone 5 mg bid) on a day before delivery and surfactant replacement (immediate after birth, 57 hours after birth) was done in our case.
Chest X-ray has been commonly used for diagnosis of PIE. Chest CT is the definitive diagnostic tool that shows characteristic appearance of a soft tissue attenuation nodule or dot-like structures in air-filled cysts.11 However, its use has been limited in ELBW infant. In our case, chest CT was taken on day 42. CT scan was not followed because of marked improvement of chest X-ray and clinical symptom.
Standard treatment strategy for PIE has not yet been established. Surgical resection was shown to be a treatment of choice for localized PIE when nonsurgical options failed.12 Lateral decubitus positioning with uninvolved side down and selective intubation of the main bronchus on the unaffected side are relatively safe and effective. In a review, 43 cases of PIE, pneumatocele, pneumothorax of all 46 cases were successfully treated by unilateral intubation. Mild and reversible complications occurred during the procedure in 52% of cases.13 In our case, lateral decubitus positioning and unilateral intubation were not beneficial to reduce PIE. Systemic steroid administration for 3 days is known to be associated with significant clinical improvement. In a retrospective case review, 7 of 10 premature infants with PIE after 3 day course of dexamethasone (0.5 mg/kg/day) showed improvement on chest X-ray and 9 of them survived to the term.14 Hydrocortisone 1 mg/kg was used on day 9, and 0.5 mg/kg twice on day 10 in our case, and we expected improvement of the gas analysis and clinical symptom, however, chest X-ray showed no improvement. HFOV with a low os-cillatory frequency may provide benefit to preterm infants with severe PIE. By applying HFOV with a low oscillatory frequency to ELBW infant, 71% of infants with bilateral emphysema and all of the infants with unilateral emphysema survived. Therefore, we applied HFOV from 12 Hz oscillation to 6 Hz gradually, keeping the low oscillatory frequency (6–8 Hz) for several days, however, there's no significant change of the PIE.
There are a few reports to describe percutaneous catheter insertion as treatment of PIE, especially in ELBW infants. Milligan, et al.15 described that 75% of premature infant with PIE were treated by chest tube insertion, Watanabe, et al.16 reported an ELBW infant (420 g) whose diffuse PIE was evacuated. And Fujii and Moulton17 reported ELBW infant (533 g) with PIE treated by pigtail catheter insertion into pneumatocele and chest tube to pleural cavity. In our ELBW infant (660 g), PIE was evacuated by insertion of central venous catheter into the PIE.
To our best knowledge, this is the first report in Korea that describes a case of ELBW infant with PIE refractory to conservative management by percutaneous catheter insertion. In summary, percutaneous catheter insertion into the PIE with consecutive tension release of the overinflated lung by drainage would be a therapeutic option for premature infants suffering from diffuse PIE.
Figures and Tables
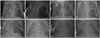
Fig. 1
Chest radiographic findings. (A) Initial finding of the PIE on day 12. (B) PIE aggravated on day 25. (C) Decreasing size of the PIE on day 30. (D) The size of the PIE increased after stopping the drainage on day 30. (E) Tension pneumothorax on day 45. (F) Improved PIE after decompression on day 45. (G) Removal of both tubes and stopping the drainage on day 56. (H) Endotracheal extubation on day 57. PIE, pulmonary interstitial emphysema.
References
1. Morisot C, Kacet N, Bouchez MC, Rouland V, Dubos JP, Gremillet C, et al. Risk factors for fatal pulmonary interstitial emphysema in neonates. Eur J Pediatr. 1990; 149:493–495.

2. Jassal MS, Benson JE, Mogayzel PJ Jr. Spontaneous resolution of diffuse persistent pulmonary interstitial emphysema. Pediatr Pulmonol. 2008; 43:615–619.

3. Jeng MJ, Lee YS, Tsao PC, Soong WJ. Neonatal air leak syndrome and the role of high-frequency ventilation in its prevention. J Chin Med Assoc. 2012; 75:551–559.

4. Greenough A, Dixon AK, Roberton NR. Pulmonary interstitial emphysema. Arch Dis Child. 1984; 59:1046–1051.

5. Hart SM, McNair M, Gamsu HR, Price JF. Pulmonary interstitial emphysema in very low birthweight infants. Arch Dis Child. 1983; 58:612–615.

6. Bawa P, Soontarapornchai K, Perenyi A, Goldfisher R, Amodio J. Development of localized pulmonary interstitial emphysema in a late preterm infant without mechanical ventilation. Case Rep Pediatr. 2014; 2014:429797.

7. Verma RP, Chandra S, Niwas R, Komaroff E. Risk factors and clinical outcomes of pulmonary interstitial emphysema in extremely low birth weight infants. J Perinatol. 2006; 26:197–200.

8. Kim SY, Lee PS, Lee SG. Study of the risk factors for pulmonary interstitial emphysema related to mechanical ventilator care. Korean J Pediatr. 2008; 51:1179–1184.

9. Cochran DP, Pilling DW, Shaw NJ. The relationship of pulmonary interstitial emphysema to subsequent type of chronic lung disease. Br J Radiol. 1994; 67:1155–1157.

10. Polin RA, Carlo WA. Committee on Fetus and Newborn. American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014; 133:156–163.

11. Donnelly LF, Frush DP. Localized radiolucent chest lesions in neonates: causes and differentiation. AJR Am J Roentgenol. 1999; 172:1651–1658.

12. Schneider JR, St Cyr JA, Thompson TR, Johnson DE, Burke BA, Foker JE. The changing spectrum of cystic pulmonary lesions requiring surgical resection in infants. J Thorac Cardiovasc Surg. 1985; 89:332–339.

13. Joseph LJ, Bromiker R, Toker O, Schimmel MS, Goldberg S, Picard E. Unilateral lung intubation for pulmonary air leak syndrome in neonates: a case series and a review of the literature. Am J Perinatol. 2011; 28:151–156.

14. Fitzgerald D, Willis D, Usher R, Outerbridge E, Davis GM. Dexamethasone for pulmonary interstitial emphysema in preterm infants. Biol Neonate. 1998; 73:34–39.

15. Milligan DW, Issler H, Massam M, Reynolds EO. Treatment of neonatal pulmonary interstitial emphysema by lung puncture. Lancet. 1984; 1:1010–1011.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download