Abstract
Purpose
The aim of this study was to investigate whether the peroxisomal proliferator-activated receptor gamma (PPARγ) ligand troglitazone in combination with photodynamic therapy (PDT) enhances the apoptotic response of DLD-1 colon cancer cells.
Materials and Methods
The effects of troglitazone, PDT, and troglitazone in combination with PDT on cell viability and apoptosis were assessed in DLD-1 cells. Cell viability and proliferation were evaluated using the tetrazolium-based MTT assay, and apoptosis was evaluated via cell staining with propidium iodide (PI) and annexin V-FITC. The levels of pro-caspase-3 were measured via Western blot analyses.
Results
Treatment of troglitazone and PDT induced the growth retardation and cell death of DLD-1 cells in a dose-dependent manner, respectively. The combination treatment significantly suppressed cell growth and increased the apoptotic response of DLD-1 and resulted in apoptosis rather than necrosis, as shown by PI/annexin V staining and degradation of procaspase-3.
Photodynamic therapy (PDT) is a therapeutic modality using light and light-sensitive chemicals (photosensitizers). Its application has been successful in both neoplastic and non-neoplastic diseases.1 Recently, this therapy has been widely applied in various tissues and organs, including the retina, esophagus, stomach, skin, and colon. Krosl, et al.2 reported that the antitumor effect of PDT was improved by the administration of granulocyte-macrophage colony-stimulating factor (GM-CSF). In addition, certain gene transfections, such as Bcl-2 and IL-6, were reported to increase cellular sensitivity to PDT.34
Peroxisomal proliferator-activated receptor gamma (PPARγ) is a member of the nuclear receptor superfamily and plays a role in global cellular functions, including lipid and glucose metabolism, insulin sensitivity, adipocyte differentiation, proliferation, and apoptosis.5 Similar to the binding of ligands, PPARγ heterodimerizes with retinoid X receptor alpha and undergoes a conformational change that leads to the binding of a specific DNA sequence (peroxisomal proliferator response element).6 Recent reports indicate that, in addition to adipocytes, PPARγ is expressed at a significant level in various kinds of tumors, such as breast adenocarcinoma,789 colon adencarcinoma,10 liposarcoma,11 lung adenocarcinoma,12 and neuroblastoma.13 Many reports have demonstrated that activation of PPARγ by its specific ligand causes cancer cells to undergo apoptosis. It was also reported that PPARγ ligands induce growth retardation14 and apoptotic or non-apoptotic cancer cell death.15161718 However, the molecular mechanisms of PPARγ-induced apoptosis are currently under investigation.
Due to the limitation of the tolerable dose of PPARγ ligand in vivo, trials have been required to use a combination therapy with another cytotoxic drug. For example, the combination of PPARγ ligand with TNF-related apoptosis-inducing ligand (TRAIL) demonstrated an improved killing effect on cancer cells.1920 With this background, we asked whether treatment using troglitazone improves PDT tumor responsiveness and addressed this question by analyzing cell proliferation and apoptosis among DLD-1 colon cancer cells. Our results indicate enhanced tumoricidal activity when PDT is combined with the PPARγ ligand troglitazone.
DLD-1, HCT-15, and HT-29 cells (human epithelial colon cancer cell lines, ATCC CCL 221) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA). Pyropheophorbide-a methyl ester (PPME) was solubilized in N, N-dimethyl formamide (DMF). Troglitazone was provided from SanKyo (Tokyo, Japan) and adjusted to 10 mM in 19% (w/v) bovine serum albumin (BSA) and 5% (v/v) dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and added to the cell culture in a final concentration of 1 to 100 µM. Added to the control cultures was the same amount of DMSO with BSA, which was less than 0.2% (v/v) of the culture medium.
Total RNA was prepared using an RNeasy Kit (Qiagen Inc., Chatsworth, CA, USA). cDNA was synthesized from 5 µg of total RNA using 2 µg random hexamer (Amersham Pharmacia Biothech Inc., Uppsala, Sweden), 1.25 mM dNTP (Boehringer-Mannheim, Mannheim, Germany) and 200 U M-MLV reverse transcriptase (Gibco BRL). PCR was performed using 0.25 mM dNTP, 0.25 U Taq polymerase (Perkin Elmer, Norwalk, CT, USA), 10 pmole primer pair, and 3 µL cDNA with a thermal cycler (Perkin Elmer). The primer sequences were as follows: PPARγ, 5'-TCCGTGATGGAAGACCACTC-3' (sense, 190–209) and 5'-CCCTTGCATCCTTCACAAGC-3' (antisense, 502–521; GeneBank U09138); and β-actin, 5'-TTGTAACCAACTGGGAC GATTGG-3' (sense, 1552–1575) and 5'-GATCTTGATCTTCATG GTGCTAGG-3' (antisense, 2967–2991; GeneBank J00691). PCR cycling conditions were as follows: 92℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 1 min.
For photodynamic analysis, target cells (1×106 in a 35-mm2 dish) were pre-incubated with 1.5 mL of RPMI added with varied doses of PPME (0.1–1 µg/mL) for 1 h at 37℃. Following incubation, the cells were exposed to light. The light source was a 200-W halogen lamp (MVI, Micro Video Instruments Inc., Avon, MA, USA) attenuated by a 515-nm filter. The total power output for the irradiation of the cells was adjusted to 120 mJ/cm2 as indicated by a Laser power meter (Metrologic Instruments, Inc., Blackwood, NJ, USA). Then, cells treated with PPME/PDT were further incubated at 37℃ for 24 h. Cells were stained with propidium iodide (PI) and annexin V-FITC (Biosource International, Camarillo, CA, USA), and cell death was analyzed via flow cytometry using the FACScan flow cytometer (Becton-Dickinson, CA, USA).
Cells (1×103 to 3×103) were cultured in 96-well plates with or without troglitazone for various concentrations. After incubation (1, 2, 4, and 6 days respectively), cells were washed in phosphate-buffered saline (PBS, pH 7.4), added to 50 µL of the 1-mg/mL MTT solution in sterile PBS, and incubated for 4 h at 37℃. Then, the plate was centrifugated at 3000 rpm for 10 min, supernatant was carefully removed, and remaining adhered cells were dissolved in 50 µL DMSO (Sigma). The plate was read at 570 nm using an ELISA reader (Softmax Pro, Biorad, Hercules, CA, USA).
Cells were cultured in 6-well plates with or without 50 µM troglitazone and PDT and then lysed with lysis buffer containing 50 mM Tris-HCl (pH 7.4), 100 mM NaCl, 0.5 mM EDTA, 0.2% NP-40, and 2 mM MgCl2 supplemented with protease inhibitor cocktail (Sigma). The samples were analyzed via 10% SDS-polyacrylamide gel electrophoresis. After transfer, membranes were blocked with 5% non-fat dry milk in PBS (w/vol)-0.05% Tween 20 and incubated overnight at 4℃ with anti-caspase-3 Ab (Transduction Lab., Lexington, KY, USA), anti-cytochrome-C Ab (Pharmingen, San Diego, CA, USA), and anti-α-tubulin Ab (Oncogene, Boston, MA, USA). Horseradish peroxidase-conjugated goat anti-mouse antibody (DAKO, Glostrup, Denmark) was used at a 1:1000 dilution as the secondary antibody, and reactive proteins were detected by incubation in SuperSignal substrate (Pierce, Rockford, IL, USA) followed by exposure to X-ray film (Eastman Kodak Company, Rochester, NY, USA). For quantification, densities of procaspase-3 bands were normalized to the density of cytochrome C bands measured using Image J software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA; available at http://rsb.info.nih.gov/ij/index.html).
To test the mRNA expression of PPARγ in DLD-1 cells, we performed RT-PCR. Consistent with previous reports,21 PPARγ mRNA was detected in DLD-1 (Fig. 1). To test whether troglitazone influences the proliferation of DLD-1 cells, an MTT assay was performed. As shown in Fig. 1, the PPARγ agonist, troglitazone, had almost no effect on the growth of DLD-1 cells during the first 2 days of the culture, although it significantly inhibited the expansion of DLD-1 cells after 4 days in a troglitazone dose-dependent manner. A dose of 100 µM troglitazone reduced cell survival and induced a morphological change of DLD-1 (data not shown). The number of cells recovered from the group treated with 50 µM troglitazone on day 6 was threefold less than that of the control, suggesting that troglitazone effectively blocks the proliferation of DLD-1 cells.
To test the effects of PDT on the cell viability of DLD-1, cell viability was measured via MTT assay 24 h after PDT with various concentrations of PPME. PDT reduced the viability of DLD-1 in a PPME dose-dependent manner (Fig. 2A). To determine the photosensitizer uptake in the cells, DLD-1 cells were incubated with 200 nM PPME for 1 h, and the uptake of PPME was detected via fluorescent microscopy. Fig. 2B showed a strong uptake of photosensitizer in the cytoplasm of DLD-1 cells. Using a light microscope, we observed floating and membrane blebbing of DLD-1 after 0.4 µg PPME/PDT, indicating that PPME/PDT induced the cellular change of apoptosis (Fig. 2C).
To test whether PPARγ enhances PDT-induced apoptosis, we examined the effect of PPARγ activation on the death of tumor cells using troglitazone and PDT. DLD-1 cells were pre-incubated with 10 mM troglitazone for 24 h and then treated with PDT. As shown in Fig. 3A, 0.4 µg/120 mJ PDT reduced cell viability by approximately 60%. This effect was augmented by pre-incubating cells with 20 mM troglitazone for 24 h. On using HCT-15 and HT-29 cells, we also observed a similar result of decreased cell viability in response to combination treatment (Fig. 3B). These results suggest that the PPARγ ligand, troglitazone, enhances the sensitivity of colon cancer cells to PDT-induced cell death.
PDT can induce either apoptotic or necrotic cell death. Generally, high-dose PDT induces cancer cell necrosis immediately, while low doses show delayed-type apoptosis, rather than necrosis. In our system, cells treated with over 0.5 µg/mL photosensitizer underwent necrotic cell death. To test whether combination treatment induces apoptosis or necrosis, we performed a flow cytometric analysis using the Annexin V/PI staining method, which is commonly used to differentiate necrotic and apoptotic cell populations. As shown in Fig. 4, troglitazone plus PDT showed a drastic increase in apoptotic cell population compared to other treatments.
Caspase activation is an important event in the apoptotic pathway and can serve as a marker of apoptosis. Given that caspase-3 is a central executioner caspase, we performed a Western blot analysis with anti-caspase-3 antibody. As shown in Fig. 5, procaspase-3 was reduced in the combination treatment with PDT plus troglitazone. This result suggests that the combination of PDT and troglitazone significantly induces the degradation of procaspase-3 and leads to apoptosis, in agreement with data in Fig. 4. It is tempting to speculate, therefore, that the combination of PDT and troglitazone is a potent killing modality in DLD-1.
PDT induces apoptosis in a variety of tumor cell lines yet does not induce apoptosis among most normal cells. It has been clinically applied in treatments of head and neck, brain, bladder, skin, and intrathoracic malignancies.1 PPARγ ligands inhibit the proliferation of colon cancer cells and induce apoptotic or non-apoptotic cell death in various tumors. In this study, we investigated the effect of the PPARγ ligand troglitazone on the efficiency of PDT in DLD-1 colon cancer cells. Our data showed that troglitazone not only inhibited the proliferation but also sensitized DLD-1 to PDT-induced cell death. Whereas no direct cytotoxicity was observed in low-dose treatments of troglitazone (<50 µM), high-dose treatments resulted in a toxic effect on DLD-1 cells. The effect of PDT is presented in Fig. 3 and we selected a PDT dose of 0.4 µg/120 mJ for IC50. Using a combination of PDT and low-dose troglitazone, we minimized immediate necrotic cell death. Although no inhibitory effect was shown in a single treatment, the combination of PDT with troglitazone effectively killed DLD-1 cells. DLD-1 underwent apoptosis rather than necrosis, as shown in Fig. 4, and caspase-3 might also be involved in PDT/troglitazone-induced apoptosis (Fig. 5).
Although PDT has tumoricidal activity, the cellular mechanism responsible for the tumor selectivity was not completely clarified. As a tumor killing mechanism, it is reported that PDT invokes the production of reactive oxygen species (ROS), and this kills tumor cells successfully. 222324 Other data indicate that certain kinds of photosensitizers directly target mitochondria, inducing apoptosis by releasing cytochrome C and activating various caspases.252627 Recently, Matroule, et al.28 reported that HCT-116 underwent apoptosis via PDT and that mitochondria and ROS played a central role in PPME-mediated apoptosis. Tumoricidal activity of PDT has also been reported in many types of cancer cell lines as well as colon cancer cell lines.26 According to a previous report, PDT induces activation of NF-κB as well as an apoptotic pathway.28 It was suggested that the activation of NF-κB may involve restoration from a death pathway after PDT. Among the various effects of PPARγ ligands, they are particularly known to antagonize the activities of several transcription factors including AP-1, STAT, and NF-κB.293031 Our data demonstrate that combination of PDT and troglitazone increases the degree of apoptosis and degradation of procaspase-3, raising the possibility that troglitazone effectively inhibits the activation of the survival pathway after PDT.
In addition, it has been reported that PPARγ regulates the expression of cyclin-dependent kinase inhibitors p18 and p21, inducing growth arrest and differentiation.3233 It may be possible that pretreatment of troglitazone may increase the susceptibility to PDT via disturbance in cell cycle regulation. However, the sensitizing mechanism of troglitazone in PDT-induced apoptosis remains to be determined.
PDT induces apoptosis as well as necrosis in cancer cells. Our data demonstrate for the first time that troglitazone treatment enhances PDT-induced cytotoxicity in DLD-1 cells, and these results suggest a new therapeutic tool for combination treatment that is free of severe side effects. However, it must be further investigated whether a combination of PDT with PPARγ agonist is safe in animals, and many experiments will be required. In summary, our study resulted in a novel observation of troglitazone sensitizing PDT-induced apoptosis, which indicates that this combination may be an effective modality for the treatment of colon cancer.
Figures and Tables
 | Fig. 1Inhibition of DLD-1 proliferation by troglitazone. DLD-1 cells were seeded in a 96-well plate at a concentration of 1×104 cells/mL, and growth was analyzed at the indicated day via MTT assay. The small box represents the mRNA expression level of DLD-1, and preadipocytes were used as positive controls. Results are presented as the mean±SD of three independent experiments performed with triplicate samples. *p<0.001. D, DLD-1 cells; P, preadipocyte. |
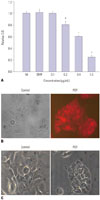 | Fig. 2Induction of DLD-1 cell death by PDT. (A) Cell death was induced by various doses of photosensitizer/PDT. DLD-1 cells (3×104 cells/mL) were placed in a 96-well plate, and PDT and MTT assays were performed as described in the Materials and Methods section. (B) Rapid uptake of photosensitizer in the cytoplasm of DLD-1. DLD-1 cells were loaded with 200 nM PPME for 1 h and subsequently detected using a fluorescent microscope at 650 nm. (C) Morphological change of DLD-1 after PDT was observed using a light microscope. *p<0.01, †p<0.001. M, medium only; DMF, N, N-dimethyl formamide; Control, DMF plus 120 mJ light; PDT, photosensitizer in DMF plus 120 mJ; PDT, photodynamic therapy; PPME, pyropheophorbide-a methyl ester. |
 | Fig. 3Enhancement of PDT-induced cell death by the pre-incubation of troglitazone. DLD-1, HCT-15, and HT-29 cells were treated with 20 µM troglitazone for 24 h and then treated to 0.4 µg/120 mJ PDT. After incubation for 24 h, cell viability was detected via MTT assay. *p<0.01, †p<0.001. M, solvent control only; Tro (T), troglitazone only; PDT (P), PDT only; Tro+PDT (T+P), combination of PDT and troglitazone, PDT, photodynamic therapy. |
 | Fig. 4Combination treatment induced apoptosis. After treatment as indicated, apoptotic cell death was detected with Annexin V/PI staining as described in the Materials and Methods section. The data represent three independent experiments. PDT, photodynamic therapy. |
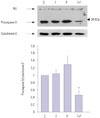 | Fig. 5Combination treatment induced procasepase-3 degradation after the pretreatment of troglitazone for 24 h, followed by incubation with or without PDT for an additional 24 h, using the same method as in Figs. 3 and 4. Whole cell lysate was compensated by BCA reagent, and an equal amount was analyzed using specific antibody as indicated. Approximately 34 KDa of caspase-3 precursor were detected. Nonspecific binding (NS) and total cytochrome C are shown as equivalent loading. Pro-caspase-3 levels were quantified densitometrically and normalized to the level of cytochrome C. The control level of procaspase-3 expression was assigned a value of 1. Data are expressed as mean values±SEM of two independent samples. *p<0.05 vs. untreated control. C, solvent control only; T, troglitazone only; P, PDT only; T+P, combination of PDT with troglitazone; PDT, photodynamic therapy. |
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by Korean Government (MSIP) Grant 2012R1A5A2A32671866.
References
1. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998; 90:889–905.

2. Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996; 56:3281–3286.
3. Kim HR, Luo Y, Li G, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999; 59:3429–3432.
4. Usuda J, Okunaka T, Furukawa K, Tsuchida T, Kuroiwa Y, Ohe Y, et al. Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer. 2001; 93:475–480.

5. Vanden Heuvel JP. Peroxisome proliferator-activated receptors: a critical link among fatty acids, gene expression and carcinogenesis. J Nutr. 1999; 129:2S Suppl. 575S–580S.

6. Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000; 5:545–555.

7. Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998; 1:465–470.
8. Badawi AF, Badr MZ. Expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-gamma and levels of prostaglandin E2 and 15-deoxy-delta12,14-prostaglandin J2 in human breast cancer and metastasis. Int J Cancer. 2003; 103:84–90.

9. Kim KY, Kim SS, Cheon HG. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem Pharmacol. 2006; 72:530–540.

10. DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998; 19:49–53.

11. Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997; 94:237–241.

12. Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000; 60:1129–1138.
13. Han S, Wada RK, Sidell N. Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferator-activated receptor gamma. Cancer Res. 2001; 61:3998–4002.
14. Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998; 4:1046–1052.

15. Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, et al. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001; 15:2007–2009.

16. Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000; 11:49–61.
17. Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000; 270:400–405.

18. Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007; 18:237–244.

19. Göke R, Göke A, Göke B, Chen Y. Regulation of TRAIL-induced apoptosis by transcription factors. Cell Immunol. 2000; 201:77–82.

20. Göke R, Göke A, Göke B, El-Deiry WS, Chen Y. Pioglitazone inhibits growth of carcinoid cells and promotes TRAIL-induced apoptosis by induction of p21waf1/cip1. Digestion. 2001; 64:75–80.

21. Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn J Cancer Res. 1999; 90:75–80.

22. Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR, Oleinick NL. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991; 51:5993–5996.
23. Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001; 276:47379–47386.
24. Luo Y, Chang CK, Kessel D. Rapid initiation of apoptosis by photodynamic therapy. Photochem Photobiol. 1996; 63:528–534.

25. Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001; 84:1099–1106.

26. Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998; 437:5–10.

27. Musser DA, Oseroff AR. The use of tetrazolium salts to determine sites of damage to the mitochondrial electron transport chain in intact cells following in vitro photodynamic therapy with Photofrin II. Photochem Photobiol. 1994; 59:621–626.

28. Matroule JY, Carthy CM, Granville DJ, Jolois O, Hunt DW, Piette J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene. 2001; 20:4070–4084.

29. Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998; 273:25573–25580.

30. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998; 391:79–82.

31. Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000; 97:4844–4849.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download