Abstract
Purpose
To assess the ability of a mechanical in-exsufflator (MI-E), either alone or in combination with manual thrust, to augment cough in patients with neuromuscular disease (NMD) and respiratory muscle dysfunction.
Materials and Methods
For this randomized crossover single-center controlled trial, patients with noninvasive ventilator-dependent NMD were recruited. The primary outcome was peak cough flow (PCF), which was measured in each patient after a cough that was unassisted, manually assisted following a maximum insufflation capacity (MIC) maneuver, assisted by MI-E, or assisted by manual thrust plus MI-E. The cough augmentation techniques were provided in random order. PCF was measured using a new device, the Cough Aid.
Results
All 40 enrolled participants (37 males, three females; average age, 20.9±7.2 years) completed the study. The mean (standard deviation) PCFs in the unassisted, manually assisted following an MIC maneuver, MI-E-assisted, and manual thrust plus MI-E-assisted conditions were 95.7 (40.5), 155.9 (53.1), 177.2 (33.9), and 202.4 (46.6) L/min, respectively. All three interventions significantly improved PCF. However, manual assistance following an MIC maneuver was significantly less effective than MI-E alone. Manual thrust plus MI-E was significantly more effective than both of these interventions.
Patients with neuromuscular disease (NMD) inevitably develop cough impairment due to dysfunction of the respiratory muscles.1 This is problematic, as impaired cough limits the ability of the patient to remove airway secretions.1 Cough augmentation can be achieved via several manual and mechanical methods.2 One such method involves the use of a mechanical in-exsufflator (MI-E), which gradually inflates the lungs (insufflation), followed by an immediate and abrupt change to negative pressure. This produces a rapid exhalation (exsufflation), which simulates a cough and thus moves secretions cephalad.3 Manually assisted coughing following maximum insufflation by air stacking is also an effective method for improving peak cough flow (PCF) in NMD patients, as reported by numerous studies.4567
However, additional methods that improve PCF further are needed. One possibility is combining MI-E with manual thrust. To our knowledge, the additional benefit of MI-E in combination with manual thrust on the improvement of PCF has not been demonstrated in clinical practice, although several studies compared PCF created during unassisted coughing, during techniques involving a maximal insufflation capacity (MIC) maneuver combined with manually assisted coughing, by MI-E, and by MI-E combined with manual thrust.7
The aim of the present randomized controlled trial was to investigate the ability of manual thrust assistance following an MIC maneuver, MI-E alone, and MI-E in combination with manual thrust to improve PCF in patients with NMD and respiratory muscle dysfunction. To measure PCF objectively, a new device, the Cough Aid, was used. We previously developed and tested this device and confirmed that it accurately measures assisted PCF in patients with amyotrophic lateral sclerosis (ALS).8
In total, 40 patients (37 males, three females; average age, 20.9±7.2 years) with NMD participated in this study. The study subjects were all patients with stable NMD who were on noninvasive mechanical ventilation and familiar with MI-E at the time of enrollment. Patients were excluded if, during the study, they had pneumonia or another respiratory intercurrent infection, cognitive impairment, severe bulbar dysfunction,7 or a tracheostomy status. All participants provided a written informed consent form, and our local Ethics Committee approved the study protocol.
Forced vital capacity, maximal inspiratory pressure, and maximal expiratory pressure were measured as previously described.9
The primary outcome measure was unassisted and assisted PCF, which was measured using the Cough Aid as described previously.8 The Cough Aid consists of two parts, namely, the connection part and the control part (Fig. 1A). The connection part is a T-shaped plastic tube that has three main air pathways: a patient connection port, an insufflation port, and an exsufflation port. The patient connection port is connected to the patient's airway via an oronasal mask. The cylindrical insufflation port is connected to the MI-E or Ambu bag via a one-way valve, which does not leak (Fig. 1B). The exsufflation port is connected to the control part. If the pushing bar of the control part (Fig. 1A) is not pressed, it blocks the exhaust holes such that the air cannot pass through the control part. However, when the pushing bar is pressed by cough, the airflow can pass through the Cough Aid to the outside of the device via the opened exhaust holes, which are the only route by which air can exit from the device. In this situation, the spirometer can distinguish the airflow caused by the MI-E or Ambu bag from the airflow produced by the patient's cough, thus allowing measurement of PCF alone. During insufflation, the airstream from the MI-E or Ambu bag goes through the patient connection part entirely, and additional air can be instilled into the patient's lungs up to the MIC, as the pushing bar is not pressed. The MIC is the volume of air that can be held in the lung with the glottis closed.10
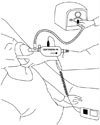
PCF was measured under four different conditions: no assistance, manual thrust following a MIC maneuver, MI-E, and MI-E in combination with manual thrust. Two investigators carried out the trial. One doctor used an Ambu bag or MI-E with the Cough Aid device, and the other doctor performed manual thrust (Figs. 2 and 3). The same doctors performed the different cough techniques and measurements for each patient. Unassisted PCF was measured by asking the subject to cough as forcefully as possible through the Cough Aid; at the same time, the pushing bar was pressed to allow air to pass through the Cough Aid.8 A commercial flow analyzer test system (Certifier® FA, TSI Inc., Shoreview, MN, USA) belonging to the Cough Aid was used to measure PCF.8 Manual-assisted PCF following an MIC maneuver was measured by first inducing maximal air stacking with an Ambu bag so that MIC was achieved while the Ambu bag was attached to the connection part of the Cough Aid and the pushing bar was not pressed.8 The patient was then asked to cough when the pushing bar was pressed; at the same time, manual abdominal thrust was applied (Fig. 2). MI-E-assisted PCF was measured after the MI-E (CoughAssist; Respironics, Inc.; Murrysville, PA, USA) was connected to the Cough Aid and set to give 40 cm H2O inspiratory pressure and -40 cm H2O expiratory pressure.11 The MI-E cycled manually, which in the present study allowed the experienced rehabilitation medicine doctor to coordinate the function of the MI-E with the patient's inspiratory and coughing efforts. The insufflation and exsufflation durations were 3 and 2 seconds, respectively. Each patient received five insufflation-exsufflation cycles of MI-E divided by 3-second inter-cycle pauses.4 On the fifth application, the subject was asked to produce a maximal voluntary cough into the Cough Aid (Fig. 3). The PCF after assistance by MI-E in combination with manual thrust was measured as described above for the MI-E-assisted PCF, and abdominal thrust was performed by the experienced rehabilitation medicine doctor to coincide with the cough. All PCF measurements were performed with the patient positioned 60 to 90 degrees from the supine position during coughing. Each of the four maneuvers was conducted at least three times, with rest periods between them. The highest values obtained in these repeated maneuvers were used for analysis.1 All participants underwent the four conditions (no assistance, manual thrust following an MIC maneuver, MI-E, and MI-E in combination with manual thrust), with a 10-minute washout period between conditions. The order of the PCF measurements was randomized.
To estimate reliability, two investigators measured the PCF using the Cough Aid in 16 normal persons. Each rater measured the PCF at three separate times at intervals of 1 day without data from previous measurements. Intrarater reliability and interrater reliability were evaluated using the intraclass correlation coefficient (ICC). Intrarater reliability was within the acceptable levels for both raters. The ICCs were found to be 0.99 and 0.98, respectively. The ICC for interrater reliability was 0.98.
The four conditions were compared in terms of PCF by using a repeated measure analysis of variance. If the repeated measure analysis of variance detected significant differences, post hoc analyses were performed by using a least significant difference (LSD) test. The analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA), and p values of less than 0.05 were regarded as statistically significant.
All enrolled participants completed the study. All three cough-augmentation techniques were well-tolerated, and the attendance rate was 100%. The demographic characteristics and pulmonary function test results including respiratory muscle strength and spirometry data for the patients are shown in Table 1. The average (standard deviation) PCF in the unassisted state was 95.7 (40.5) L/min. After manual assistance following an MIC maneuver, the PCF was 155.9 (53.1) L/min. After MI-E alone and in combination with manual thrust, the PCFs were 177.2 (33.9) and 202.4 (46.6) L/min, respectively (Table 2). The repeated measures analysis of variance indicated that these values differed significantly (F=84.92, p<0.01) (Table 2). Use of the LSD test revealed that manual assistance following an MIC maneuver, MI-E alone, and MI-E plus manual thrust significantly improved PCF relative to unassisted PCF (F=84.92, p< 0.01). Moreover, manual assistance following an MIC maneuver was significantly less effective than MI-E alone or in combination with manual thrust (F=84.92, p<0.01). MI-E plus manual thrust was significantly more effective than MI-E alone (F=84.92, p<0.01) (Table 2). The large standard deviations indicated that the PCFs were highly variable. This likely reflected the heterogeneity of the patient population in terms of disease severity.
This was the first clinical trial to demonstrate the superior ability of an MI-E in combination with manual thrust to improve PCF more so than other cough-augmentation techniques in ventilator-dependent patients with NMD who had severe degrees of PCF impairment. In addition, PCF was measured objectively by using a new device, the Cough Aid.
Our study of 40 patients with NMD showed that MI-E alone improved PCF significantly more than manual assistance following an MIC maneuver. In contrast, a recent meta-analysis failed to detect any significant differences between MI-E and other manual cough assistance techniques.5 This disparity between our study and the meta-analysis may reflect the use of different methods of assistance and measuring PCF. Moreover, the MI-E protocol used in our study (i.e., the pressures and application times) differed from the protocol used in several of the studies that were included in the meta-analyses; the other studies that were included did not consistently report thee MI-E protocol used.121314 Notably, our results are consistent with those of Bach4 in 21 patients with NMD; they found that manual assistance after maximal insufflation was not as effective as an MI-E in terms of increasing PCF. However, it should be noted that Bach did not randomize the delivery of the interventions; instead, manual assistance following an MIC maneuver was always followed by the MI-E, which may have introduced selection bias. Conversely, in the present study, the four conditions (no assistance, manual assistance following an MIC maneuver, assistance using an MI-E, and assistance using both an MI-E and manual thrust) were delivered in random order. Our study contrasts somewhat from the study of Senent, et al.,6 who examined 16 patients with ALS; they found that while the PCFs produced by manual thrust after maximum insufflation were smaller on average than those produced when an MI-E was used, this difference did not achieve statistical significance. This disparity may reflect the fact that Senent, et al. performed four to six insufflation-exsufflation cycles with 1- to 3-second inter-cycle pauses, although this protocol was apparently not applied consistently. Moreover, the insufflation and exsufflation durations were not reported. In contrast, we always performed five cycles of 3-second insufflation and 2-second exsufflation; moreover, the cycles were always interspersed by 3-second pauses. In addition, the cohorts in our and Senent, et al.'s studies differed markedly: all subjects in the study by Senent, et al. had ALS, some of whom had very severe bulbar symptoms. In contrast, all subjects in the present study had hereditary NMD, mainly Duchenne muscular dystrophy and spinal muscular atrophy. Effective coughing requires that the upper airway muscles coordinate glottis closure and opening; this process can be impaired in ALS.6 Thus, the different disease courses and outcomes of ALS and NMD indicate that studies on ALS should be considered separately from studies on hereditary NMD. In contrast with our results, in a study by Lacombe, et al.7 conducted on 18 patients with NMD, PCF was higher when using an insufflation technique combined with manual assistance than when using an MI-E combined with manual assistance or an MI-E alone. They also suggested that adding the MI-E device to manual assistance was unhelpful in patients whose PCF when using an insufflation technique and manual assistance exceeded 5 L/s, as the expiratory flow produced by the patient's effort and manual assistance transitorily exceeded the vacuum capacity of the MI-E device, which therefore became a transient load against the PCF.7 However, the conditions differed between this study and our own. In the present study, the average PCF in the unassisted state of the cohort was only 95.7±40.5 L/min, and after manual assistance following an MIC maneuver, the average PCF was 155.9±53.1 L/min (i.e., 2.6±0.9 L/s), which was much less than 5 L/s. This may in part explain the differences in the results between the studies. Cough ability increasingly declines as the disease progresses in NMD patients, and a weak cough is an important factor that contributes to respiratory morbidity.8 Increasing cough flow would improve secretion clearance, which in turn could improve the clinical outcomes of patients with NMD in terms of morbidity and possibly survival.4 Thus, it is important that the MI-E in combination with manual thrust improves cough ability more than other cough-augmentation techniques in predominantly advanced-stage NMD patients.
Bach4 hypothesized that, should MI-E be used in conjunction with the manual thrust technique, it would tend to decrease the transtracheal pressure gradient; this in turn would cause the airway to collapse somewhat during exsufflation, thereby enhancing airway secretion elimination. However, whether an MI-E combined with manual thrust actually does improve PCF was not confirmed in a controlled clinical setting until the present study, although the combined approach was used in a previous study.715 We indeed found that in noninvasive ventilator-dependent patients with NMD with severe respiratory muscle dysfunction, an MI-E in combination with manual thrust was markedly superior to either an MI-E alone or manual assistance following an MIC maneuver and also did not induce any adverse effects. The findings of the present study are in line with the findings of Sivasothy, et al.,12 who found that in 12 subjects with respiratory muscle weakness without scoliosis, mechanical assistance plus manual thrust significantly improved PCF relative to unassisted coughing. However, it should be noted that in their study, the mechanical insufflation and exsufflation pressures were set at 20 and -20 cm H2O, respectively, which may not be adequate as several studies show that to achieve clinical efficacy, these MI-E pressures should be set at 40 and -40 cm H2O, respectively.11161718 In addition, the MI-E protocol used by Sivasothy, et al. differed from ours in that it started with two insufflation-exsufflation cycles to aid inflation and deflation of the thorax; after a third inspiration, PCF measurements were obtained during coughing with manual thrust after the patient was disconnected from the MI-E at the end of the insufflation. In other words, they did not use mechanical exsufflation during the cough itself. However, in our study, PCF measurements were obtained during coughing with manual thrust and simultaneous exsufflation assistance derived from the MI-E.
Our study yielded several notable findings. First, we used a new device, the Cough Aid, to measure assisted PCF accurately. In the study of Lacombe, et al.,7 transient positive face mask pressure was induced by expiration produced by the combined cough effort and manual assistance during mechanical expiration in certain subjects. In that case, the MI-E device and its circuitry constituted a load against PCF. However, the Cough Aid can distinguish the airflow caused by the MI-E from the airflow produced by the patient's cough, allowing the measurement of PCF alone. Thus, the MI-E device and its circuitry could not constitute a load against PCF in any case of our study, and objective measurement of PCF was possible. Second, the additional benefit of an MI-E in conjunction with the manual thrust technique was proven in terms of the PCF response to cough assistance techniques in clinical practice. This method produced high expiratory flows without raising safety issues. Additionally, this study used a larger group of NMD patients with advanced respiratory muscle weakness in comparison to previous studies. We also used standard protocol for MI-E therapy, whereas the pressures, times, and application of the MI-E amongst the earlier studies varied and were not consistently reported.5
The present study had three limitations. First, our results must be interpreted with caution, as our patients predominantly had advanced stage NMD and thus had respiration insufficiency that required non-invasive mechanical ventilation. This may limit the generalizability of our results to other NMD populations. Second, we only investigated the immediate effect of an MI-E with or without manual thrust in a clinical setting. Whether chronic use of this approach would continue to be effective in the long term remains to be tested. Finally, our data were expressed as average results for the patient cohort. As a result, individual variations were obscured. For instance, we noticed that several of our patients had better PCFs when manual assistance following an MIC maneuver was used compared to when an MI-E alone was employed.
Our study showed that in noninvasive ventilator-dependent patients with NMD with respiratory muscle weakness, an MI-E produced on average superior PCFs compared to manual thrust after maximal insufflation. In addition, an MI-E used in conjunction with manual thrust yielded the highest PCFs by a significant margin among a range of cough-augmentation techniques.
Figures and Tables
 | Fig. 1Flow analyzer test system. When performing a cough-assistance technique, the air that is inhaled from the MI-E or Ambu bag through the patient connection part does not leak though the valve; when the patient exhales or coughs, the pushing bar is pressed at the same time, and the exhaled air is pushed through the pushing bar. (A) Disassembled view. (B) Complete view showing the inner parts. MI-E, mechanical in-exsufflator. |
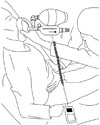 | Fig. 2Measurement of manually assisted peak cough flow following a maximum insufflation capacity maneuver. |
Table 1
Demographic Characteristics and Pulmonary Function Data for the Subjects (n=40)

Table 2
Comparison of Peak Cough Flows among Four Different Conditions

| UPCF | MPCF-MIC | PCF-MI-E | PCF-MI-EM | F value | |
|---|---|---|---|---|---|
| PCF (L/min) | 95.7±40.5 | 155.9±53.1 | 177.2±33.9 | 202.4±46.6 | 84.92* |
PCF, peak cough flow; UPCF, unassisted peak cough flow; MPCF-MIC, manual assisted peak cough flow following a maximal insufflation capacity maneuver; PCF-MI-E, mechanical insufflation-exsufflator-assisted peak cough flow; PCF-MI-EM, peak cough flow after assistance by mechanical insufflation-exsufflator in combination with manual thrust.
Values are mean±standard deviation.
*p<0.01.
References
1. Kang SW, Kang YS, Moon JH, Yoo TW. Assisted cough and pulmonary compliance in patients with Duchenne muscular dystrophy. Yonsei Med J. 2005; 46:233–238.

2. Finder JD. Airway clearance modalities in neuromuscular disease. Paediatr Respir Rev. 2010; 11:31–34.

3. Homnick DN. Mechanical insufflation-exsufflation for airway mucus clearance. Respir Care. 2007; 52:1296–1305.
4. Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993; 104:1553–1562.
5. Morrow B, Zampoli M, van Aswegen H, Argent A. Mechanical insufflation-exsufflation for people with neuromuscular disorders. Cochrane Database Syst Rev. 2013; (12):CD010044.

6. Senent C, Golmard JL, Salachas F, Chiner E, Morelot-Panzini C, Meninger V, et al. A comparison of assisted cough techniques in stable patients with severe respiratory insufficiency due to amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011; 12:26–32.

7. Lacombe M, Del Amo Castrillo L, Boré A, Chapeau D, Horvat E, Vaugier I, et al. Comparison of three cough-augmentation techniques in neuromuscular patients: mechanical insufflation combined with manually assisted cough, insufflation-exsufflation alone and insufflation-exsufflation combined with manually assisted cough. Respiration. 2014; 88:215–222.

8. Choi WA, Park JH, Kim DH, Kang SW. Cough assistance device for patients with glottis dysfunction and/or tracheostomy. J Rehabil Med. 2012; 44:351–354.

9. Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med J. 2010; 51:392–397.

10. Sancho J, Servera E, Marín J, Vergara P, Belda FJ, Bach JR. Effect of lung mechanics on mechanically assisted flows and volumes. Am J Phys Med Rehabil. 2004; 83:698–703.

11. Boitano LJ. Management of airway clearance in neuromuscular disease. Respir Care. 2006; 51:913–922.
12. Sivasothy P, Brown L, Smith IE, Shneerson JM. Effect of manually assisted cough and mechanical insufflation on cough flow of normal subjects, patients with chronic obstructive pulmonary disease (COPD), and patients with respiratory muscle weakness. Thorax. 2001; 56:438–444.

13. Chatwin M, Ross E, Hart N, Nickol AH, Polkey MI, Simonds AK. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. Eur Respir J. 2003; 21:502–508.

14. Mustfa N, Aiello M, Lyall RA, Nikoletou D, Olivieri D, Leigh PN, et al. Cough augmentation in amyotrophic lateral sclerosis. Neurology. 2003; 61:1285–1287.

15. Bach JR, Niranjan V, Weaver B. Spinal muscular atrophy type 1: a noninvasive respiratory management approach. Chest. 2000; 117:1100–1105.
16. Gómez-Merino E, Sancho J, Marín J, Servera E, Blasco ML, Belda FJ, et al. Mechanical insufflation-exsufflation: pressure, volume, and flow relationships and the adequacy of the manufacturer's guidelines. Am J Phys Med Rehabil. 2002; 81:579–583.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download