Abstract
Purpose
Progesterone resistance is thought to be a major factor that contributes to progression of endometriosis. However, it is not clear what causes progesterone resistance in endometriosis. This study aimed to assess whether cytokines or peritoneal fluid can affect progesterone receptor (PR) expression in endometrial cells and to verify whether PR expression is reduced in endometriosis.
Materials and Methods
The PR-B/A ratio was measured via real-time polymerase chain reaction after in vitro culture, in which endometrial cells were treated with either tumor necrosis factor-alpha (TNF-α), interleukin-1 beta, or peritoneal fluid obtained from women with advanced-stage endometriosis. Immunohistochemistry was performed to compare PR-B expression between eutopic and ectopic endometrial tissues from women with and without advanced-stage endometriosis.
Results
The PR-B/A ratio was significantly decreased by treatment with either TNF-α (p=0.011) or peritoneal fluid from women with advanced-stage endometriosis (p=0.027). Immunoreactivity of PR-B expression was significantly lower during the secretory phase than during the proliferative phase in endometrial tissues from control subjects (p<0.001). PR-B expression was significantly reduced in the eutopic endometrium (p=0.031) and ovarian endometrioma (p=0.036) from women with advanced-stage endometriosis compared with eutopic endometrium tissues from control subjects.
Endometriosis is a chronic disease that is characterized by endometrial cell proliferation outside of the uterine cavity, and this condition occurs in 6–10% of reproductive-age women and in 50% of infertile women.1 Although the mechanisms responsible for its pathogenesis and progression remain the subject of debate, endometriosis is widely accepted to be an estrogendependent chronic inflammatory disease.2 Indeed, medical treatment regimens that suppress estrogen levels have been effective in reducing pain and the size of the lesions, although definite treatment is not yet possible.3 Additionally, many studies have proposed that proinflammatory cytokines play a critical role in the complex inflammatory cascade that is associated with endometriosis.45678
Progesterone, a hormone that facilitates endometrial maturation for implantation, acts through the intracellular progesterone receptors (PRs). Several previous studies have suggested that a failure of progesterone to appropriately regulate gene expression during endometrial differentiation might be a critical component of the pathogenesis and/or pathophysiology of the disease.9 Kao, et al.10 showed that a group of target genes in the eutopic endometrium are normally downregulated during the window of implantation yet are significantly increased in women with endometriosis, suggesting that progesterone may mediate the expression of critical genes that could be altered in the eutopic endometrium of endometriosis patients. Specifically, Bulun, et al.11 suggested that the molecular basis of progesterone resistance in endometriosis might be associated with an overall reduction in the expression levels of PRs and the absence of PR-B, the PR isoform.
However, there have been few studies suggesting possible factors that can lead to a decreased expression of PR in endometrial cells or tissues in women with endometriosis, and it remains to be elucidated what might be the cause of progesterone resistance in endometriosis. We designed the present study for two purposes. First, we aimed to see whether proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) or peritoneal fluids obtained from women with advanced-stage endometriosis have any discernible effect on PR expression in endometrial cells. Second, we aimed to verify whether the expression of PR-B is decreased in the eutopic endometrium and ovarian endometrioma of women with advanced-stage endometriosis.
For endometrial stromal cell (ESC) cultures, endometrial samples were obtained from fertile women who were diagnosed with intramural leiomyoma at the time of hysterectomy, had no evidence of endometrial abnormalities, adenomyosis, or pelvic endometriosis, and had not taken any hormonal medication in the preceding 3 months. The endometrial samples were placed in Hank's balanced salt solution and transported to the laboratory for ESC isolation and culture. Peritoneal fluids were obtained from a total of five patients in whom advanced-stage endometriosis had been confirmed via laparoscopy before any surgical manipulation during the proliferative phase. Peritoneal fluids were centrifuged and stored at -80℃ until in vitro treatments. Written informed consent was obtained from each patient using consent forms and protocols approved by the Review Board for Human Research of Asan Medical Center.
For immunohistochemical staining, endometrial sections were obtained from a total of 32 women with carcinoma in situ (CIS) of the uterine cervix as well as a total of 39 women with histological evidence of endometriosis. All of the recruited women had regular menstrual cycles and had undergone hysterectomies via either transabdominal or laparoscopic methods. Women with an endometrial abnormality, adenomyosis, or pelvic endometriosis were excluded from the control group, and all of the women in the endometriosis group were surgically confirmed as having advanced-stage endometriosis. In the endometriosis group, the extent of disease was staged according to the American Society for Reproductive Medicine criteria.12 The date of the menstrual cycle was classified as proliferative (days 1–13), early-secretory (days 14–19), mid-secretory (days 20–23), or late-secretory (days 24–28) phase via endometrial histology using the criteria of Noyes, et al.13 Sections of endometrioma were also obtained from 28 nulliparous women in whom ovarian cystectomy was performed due to ovarian endometrioma. The clinical characteristics, the menstrual phases of each group, and the stages of endometriosis are summarized in Table 1. We did not need additional informed consent to use the specimens in this study, as only archived material was used. The Review Board for Human Research in our hospital approved this project.
TNF-α, IL-1β, and Ishikawa cell line (a well-differentiated endometrial adenocarcinoma cell line) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
ESCs were separated and maintained in a monolayer culture as described previously.14 ESCs after first passage were assayed immunocytochemically using specific cell-surface markers, and we previously showed that the purity of isolated ESCs was more than 95%.14 We utilized only the cells after the first passage in all of the experiments using ESCs.
When Ishikawa cells and ESCs were grown to 70% confluence, they were treated with serum-free, phenol red-free media (Sigma-Aldrich) for 18 h before treatment. Afterwards, cell cultures were treated with either vehicle (control), TNF-α (10 ng/mL and 25 ng/mL) or IL-1β (10 ng/mL and 25 ng/mL) for 1 h and 6 h, respectively. For treatment with peritoneal fluid, 1×105 cells were seeded in six-well plates, and when 80% confluence was reached, the cells were starved in serum-free, phenol red-free media (Sigma-Aldrich) overnight. Afterwards, either vehicle or 25% peritoneal fluid was added, and RNA was extracted 1 h and 6 h after the addition of peritoneal fluid.
Total RNA was extracted from the isolated cells using an Easy-blueTM Total RNA extraction kit (Intron Biotechnology, Seoul, Korea). RNA was measured using a Nanodrop instrument and stored at -80℃. For reverse transcription, 1 µg total RNA, 1 µL oligo d(T)15 (100 pmol/µL), and 2 µL 100 mM dithiothreitol were mixed and incubated at 70℃ for 5 min. After adding 8 µL RT-&GOTM mastermix 2.5×C (MP Biomedical, Santa Ana, CA, USA), the reverse transcripts were incubated at 70℃ for 15 min for enzymatic inactivation and then stored at -20℃.
Real-time PCR was performed using iQTM SYBR® Green supermix (BIO-RAD, Hercules, CA, USA). The primer sequences for PR-A and PR-B were as follows: PR-A forward, 5' GAGCACTGGATGCTGTTGCT 3', PR-A reverse 5' GGCTTA GGGCTTGGCTTTC 3', PR-B forward, 5' TGGGATCTGAGAT CTTCGGAG 3', and PR-B reverse, 5' GAAGGGTCGGACTTCT GCTG 3'. The iCycler (My iQTM Single Color Real-time PCR Detection System, BIO-RAD) was used for PCR with the following thermocycling conditions: 95℃ for 3 min, 95℃ for 15 sec, 59℃ for 30 sec, 70℃ for 40 sec (40 cycles), and 95℃ for 1 min.
Formalin-fixed paraffin-embedded samples were cut into 4-µm thick sections. After deparaffinization, immunohistochemical staining was performed as described in our previous study14 using a Progesterone Receptor Ab-2 primary antibody (Clone hPRa 2; 1:100; Lab vision Corporation, Fremont, CA, USA) and a biotinylated anti-mouse secondary antibody (1:400; Vector Laboratory, Burlingame, CA, USA). The intensity of PR-B immunoreactivity was semiquantitatively evaluated and analyzed as described previously.14
All of the data were normally distributed as assessed by the Kolmogorov-Smirnov test. Student's t-test was used for comparisons of two variables, and an ANOVA and the post hoc Tukey test for pairwise comparisons were used for the comparison of three variables. Statistical analyses were performed using Statistical Programs for the Social Sciences (SPSS, version 14.0, SPSS Inc., Chicago, IL, USA) software. Statistical significance was defined as p<0.05.
The PR-B/A ratios were assessed using real-time PCR in Ishikawa cells (n=5) and ESCs (n=5) following treatment with vehicle, TNF-α (10 or 25 ng/mL), or IL-1β (10 or 25 ng/mL) for 1 h or 6 h. The PR-B/A ratio was significantly lower in Ishikawa cells treated with 10 ng/mL (p=0.021) and 25 ng/mL (p=0.011) TNF-α at 1 h compared to those treated with vehicle, respectively (Fig. 1A and B). ESCs treated with 25 ng/mL TNF-α had a significantly lower PR-B/A ratio at 1 h than cells treated with vehicle (p=0.027) (Fig. 1C). However, we could not find any differences in the PR-B/A ratio following IL-1β treatment (10 or 25 ng/mL) in Ishikawa cells (Fig. 1B) as well as ESCs (Fig. 1D).
To investigate the effects of peritoneal fluid obtained from patients with advanced-stage endometriosis, the PR-B/A ratio was assayed in Ishikawa cells (n=6) and ESCs (n=6) after treatment with vehicle or peritoneal fluid for 1 or 6 h. The PR-B/A ratio was significantly reduced in Ishikawa cells treated with peritoneal fluid at 1 h (p=0.011) and at 6 h (p<0.001) compared to cells treated with vehicle, respectively (Fig. 1E and F). The ESCs treated with peritoneal fluid had a significantly lower PR-B/A ratio at 1 h than cells treated with vehicle (p=0.027).
We observed strong immunoreactivities of PR-B in the glandular and stromal cells during the proliferative phase, whereas weak or no staining of PR-B was detected during the secretory phase (Fig. 2A). On comparing the HSCOREs between the two phases, we found obvious reductions in PR-B expression during the secretory phase in the glandular cells (p<0.001) as well as in the stromal cells (p=0.001) (Fig. 2B).
Fig. 3 shows the expression of PR-B in the eutopic endometrium of controls and endometriosis patients. The immunoreactivity of PR-B was obviously decreased in the glandular cells as well as in the stromal cells of the eutopic endometrium of women with endometriosis compared to the controls (Fig. 3A and B). In the glandular cells, we observed a significant decrease of PR-B expression in the eutopic endometrium of women with endometriosis compared to the controls throughout the menstrual phases (p=0.031) as well as during the proliferative phase (p<0.001) (Fig. 3C). In the stromal cells, the immunoreactivity of PR-B was significantly lower in the eutopic endometrium of women with endometriosis than in the controls throughout the menstrual phases (p=0.001), during the proliferative phase (p=0.001), and during the early secretory phase (p<0.001), respectively (Fig. 3D).
Based on our findings that PR-B expression was lower in the eutopic endometrium of women with endometriosis than in the controls, we evaluated whether there was also any difference of PR-B expression in ovarian endometrioma. We found that the PR-B expression was significantly decreased in the glandular cells of ovarian endometrioma compared to the controls (p=0.036) (Fig. 4). However, there was no difference in PR-B expression in the stromal cells between the two groups.
We previously reported that the expression levels of p21-activated kinase (Pak)-1 and Pak-4, which have been shown to be involved in cancer development and progression, are increased in the eutopic endometrium as well as in ovarian endometrioma in women with advanced-stage endometriosis.141516 In those previous studies, we found that the expression levels of Pak-1 and Pak-4 are downregulated by in vitro treatment of progesterone in normal endometrial cells. Moreover, the immunoreactivities of both kinases are also reduced during the secretory phase when the serum progesterone level is elevated in normal endometrium of women without endometriosis. We suggested that resistance against the activities of progesterone may cause persistently high expression of Pak-1 and Pak-4 in eutopic endometrium as well as ovarian endometrioma in patients with endometriosis. Based on the findings that in vitro treatment of endometrial cells with proinflammatory cytokines resulted in increased expression of Pak-1 and Pak-4, we also proposed that the peritoneal proinflammatory microenvironment due to presence of endometrioma may drive progesterone resistance in these patients.
To the best of our knowledge, this is the first study to demonstrate that the PR-B/A ratio is significantly decreased by treatment with TNF-α and peritoneal fluid obtained from women with advanced-stage endometriosis. These findings suggest that the progesterone resistance that occurs in women with endometriosis may be a consequence of the proinflammatory peritoneal microenvironment due to the presence of endometrioma in the pelvic cavity. When considered along with our previous studies,141516 the increased levels of proinflammatory cytokines may cause a decreased PR-B/A ratio, which might in turn result in the activation of other signaling pathways to facilitate the development and progression of endometriosis as a consequence of less responsiveness to progesterone, which would normally inhibit those pathways. However, further studies are necessary to evaluate whether additional critical factors exist that would cause progesterone resistance in patients with endometriosis. Indeed, crosstalk between PR and the AKT signaling pathway has been suggested, and it has been shown that AKT is sufficient to downregulate the PR protein expression level.17
Regarding the level of PR expression in endometriosis, inconsistent data have been reported by several investigators. Attia, et al.18 found that the PR-B protein is not detectable in endometriotic tissue, whereas other studies have suggested that the level of PR expression may be unpredictable or increased in endometriosis.1920 Utilizing immunohistochemistry, we found that the immunoreactivity of PR-B was obviously decreased in the eutopic endometrium and ovarian endometrioma of women with advanced-stage endometriosis compared to the controls, which seems to be quite consistent with the data of Bulun, et al.11 and Attia, et al.18
There were several limitations in the present study. As the level of PR-B expression was not consistently assessed via Western blot analysis in an endometrial cell culture model, we were only able to compare the PR-B/A ratio using real-time PCR, similar to a previous study on PR expression level.21 We could not use endometrial tissues of absolutely disease-free controls; thus, we recruited women with CIS of the uterine cervix as controls. Despite these limitations, the present study showed for the first time that the PR-B/A ratio was significantly reduced after treatment with TNF-α or peritoneal fluid obtained from women with advanced-stage endometriosis. This study also verified the reduced expressions of PR-B in the eutopic endometrium and ovarian endometrioma of women with advancedstage endometriosis. These findings strongly suggest that progesterone resistance in endometriosis may be induced by proinflammatory conditions in the pelvic peritoneal microenvironment. Further studies are necessary to evaluate the detailed signaling pathways including nuclear factor kappa-B activation, in which proinflammatory stimuli drive progesterone resistance through multiple steps in endometrial cells.
Figures and Tables
 | Fig. 1Effects of tumor necrosis factor-alpha, interleukin-1 beta, and peritoneal fluid obtained from patients with advanced-stage endometriosis on the ratio of progesterone receptor-B (PR-B) to progesterone receptor-A (PR-A) expression in Ishikawa cells (A, B, and E) and endometrial stromal cells (C, D, and F) at 1 h and 6 h. Results on the graph are expressed as percent, where cells treated with vehicle are normalized to 100%. *p=0.021 and 0.011 vs. vehicle, †p=0.027 vs. vehicle, ‡p=0.011 and <0.001 vs. vehicle, §p=0.027 vs. vehicle. Control, vehicle; T10, tumor necrosis factor-alpha 10 ng/mL; T25, tumor necrosis factor-alpha 25 ng/mL; I10, interleukin-1 beta 10 ng/mL; I25, interleukin-1 beta 25 ng/mL. |
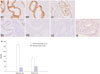 | Fig. 2Representative micrographs (A) (×200) of progesterone receptor-B immunostaining in the eutopic endometrium of the control subjects throughout the menstrual cycle and HSCOREs (B). Data of HSCOREs are expressed as mean±SEM. *p<0.001 vs. control, †p=0.001 vs. control. EP, early proliferative phase; MP, mid-proliferative phase; LP, late-proliferative phase; ES, early-secretory phase; MS, mid-secretory phase; LS, late-secretory phase; N, negative control. |
 | Fig. 3Representative micrographs of progesterone receptor-B immunostaining in the eutopic endometrium of the controls and patients with endometriosis (A: control subject, B: endometriosis patient) (×400) and HSCOREs (C: glandular cells, D: stromal cells). Data of HSCOREs are expressed as mean±SEM. *p=0.031 and <0.001 vs. control, †p=0.001, 0.001, and <0.001 vs. control. W, whole menstrual phases put together; P, proliferative phase; S, secretory phase; ES, early secretory phase; MS, mid-secretory phase; LS, late secretory phase. |
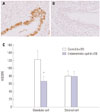 | Fig. 4Representative micrographs of progesterone receptor-B immunostaining in the eutopic endometrium of the controls and ovarian endometrioma (A: control subject, B: ovarian endometrioma) (×400) and HSCOREs (C). Data of HSCOREs are expressed as mean±SEM. *p=0.036 vs. control. |
Table 1
Clinical Characteristics of Patients and Controls

ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education (2013R1A1A2008247).
References
1. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997; 24:235–258.

3. Kim SH, Chae HD, Kim CH, Kang BM. Update on the treatment of endometriosis. Clin Exp Reprod Med. 2013; 40:55–59.

4. Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril. 1988; 50:573–579.

5. Hill JA, Anderson DJ. Lymphocyte activity in the presence of peritoneal fluid from fertile women and infertile women with and without endometriosis. Am J Obstet Gynecol. 1989; 161:861–864.

6. Fakih H, Baggett B, Holtz G, Tsang KY, Lee JC, Williamson HO. Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 1987; 47:213–217.

7. Mori H, Sawairi M, Nakagawa M, Itoh N, Wada K, Tamaya T. Peritoneal fluid interleukin-1 beta and tumor necrosis factor in patients with benign gynecologic disease. Am J Reprod Immunol. 1991; 26:62–67.

8. Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJ. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum Reprod. 1995; 10:2773–2780.

9. Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005; 83:529–537.

10. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003; 144:2870–2881.

11. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006; 248:94–103.

12. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997; 67:817–821.
13. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975; 122:262–263.

14. Kim SH, Lee HW, Kim YH, Koo YH, Chae HD, Kim CH, et al. Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum Reprod. 2009; 24:1133–1141.

15. Lee MY, Kim SH, Ihm HJ, Chae HD, Kim CH, Kang BM. Up-regulation of p21-activated kinase 1 by in vitro treatment with interleukin 1-beta and its increased expression in ovarian endometriotic cysts. Fertil Steril. 2011; 96:508–511.

16. Kim SH, Kim SR, Ihm HJ, Oh YS, Chae HD, Kim CH, et al. Regulation of P21-activated kinase-4 by progesterone and tumor necrosis factor-α in human endometrium and its increased expression in advanced-stage endometriosis. J Clin Endocrinol Metab. 2013; 98:E238–E248.

17. Lee II, Kim JJ. Influence of AKT on progesterone action in endometrial diseases. Biol Reprod. 2014; 91:63.

18. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000; 85:2897–2902.

19. Lessey BA, Metzger DA, Haney AF, McCarty KS Jr. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertil Steril. 1989; 51:409–415.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download