Abstract
Purpose
Materials and Methods
Results
Figures and Tables
 | Fig. 1Change in clinical severity of atopic dermatitis from baseline to 12 months after subcutaneous allergen immunotherapy with house dust mites extract in patients with atopic dermatitis (n=144) who received allergen immunotherapy with house dust mite extract for 12 months (A), and the degrees of clinical improvements in patients with mild to moderate atopic dermatitis and patients with severe atopic dermatitis who received allergen immunotherapy with house dust mite extract for 12 months, at 12 months compared to baseline (B). Data are expressed as means±standard error of the mean. Clinical severity score was expressing as standardized clinical severity scoring system for atopic dermatitis (SCORAD) value. Severe atopic dermatitis was defined as a baseline SCORAD value above 50. |
Table 1
Baseline Clinical Characteristics of 251 Patients with Atopic Dermatitis Who Received Subcutaneous Allergen Immunotherapy with House Dust Mite Extract

Table 2
Degree of Clinical Improvement in 144 Patients with Atopic Dermatitis Who Received Subcutaneous Allergen Immunotherapy with House Dust Mite Extract for 12 Months, at 12 Months Compared to Baseline

Table 3
Comparison of Baseline Clinical and Laboratory Parameters between Patients Showing a Favorable Clinical Response (Responders) and Patients Showing No Significant Clinical Response (Non-Responders) at 12 Months among Patients with Atopic Dermatitis Who Received Subcutaneous Allergen Immunotherapy with House Dust Mite Extract for 12 Months
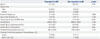
SCORAD, standardized clinical severity scoring system for atopic dermatitis; D. pteronyssinus, Dermatophagoides pteronyssinus; D. farinae, Dermatophagoides farinae.
Data are expressed as means±SD or numbers with percentages.
*p<0.05 is less than 0.05, †Responders: patients with atopic dermatitis showing a favorable clinical response to subcutaneous allergen immunotherapy as a decrease clinical severity score at 12 months greater than 50% compared to baseline value.
Table 4
Comparison of Baseline Clinical and Laboratory Parameters between Patients Showing a Favorable Clinical Response (Responders) and Patients Showing No Significant Clinical Response (Non-Responders) at 12 Months among Patients with Mild to Moderate Atopic Dermatitis and Patients with Severe Atopic Dermatitis Who Received Subcutaneous Allergen Immunotherapy with House Dust Mite Extract for 12 Months

D. pteronyssinus, Dermatophagoides pteronyssinus; D. farinae, Dermatophagoides farinae.
Data are expressed as means±SD or numbers with percentages.
*p<0.05 is less than 0.05, †Severe atopic dermatitis was defined as a baseline standardized clinical severity scoring system for atopic dermatitis (SCORAD) value above 50, ‡Responders: patients with atopic dermatitis showing a favorable clinical response to subcutaneous allergen immunotherapy as a decrease clinical severity score at 12 months greater than 50% compared to baseline value.
Table 5
Change in Laboratory Parameters in Patients with Atopic Dermatitis Who Received Subcutaneous Allergen Immunotherapy with House Dust Mite Extract from Baseline to 12 Months





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download