Abstract
Purpose
To determine the window of time during which osteoporosis affects the management of spinal surgery and the mechanism of bone metabolism changes in males with osteoporosis by examining changes in bone metabolism in young castrated male rats.
Materials and Methods
A total of 30 Sprague-Dawley rats were randomly allocated into two study groups. Group 1 (control) received a sham surgery and Group 2 received bilateral orchiectomy to change bone mineral density (BMD). Serum osteocalcin, alkaline phosphatase (ALP), and collagen type 1 cross-linked C-telopeptide (CTX) were analyzed at postoperative date (POD) 8, 10, and 12 weeks. BMDs were measured using micro computed tomography scans.
Results
Femoral and lumbar BMDs were decreased in the orchiectomy groups. BMDs in the sham and orchiectomy groups showed statistically differences at POD 8, 10, and 12 weeks for the femur (p=0.032, 0.008, 0.008) and lumbar spine (p=0.151, 0.008, 0.008, respectively). Serum osteocalcin, ALP, and CTX decreased gradually; however, N-terminal type 1 procollagen (P1NP) showed a slight increase yet no significant change.
Conclusion
In young castrated male rats, a significant decrease in BMD was observed after orchiectomy due to the mixture of two detrimental factors. Young castrated male rats did not reach peak BMD. Increased bone turnover causes bone resorption to exceed bone formation. This study may contribute to the creation of a valuable model for studies of male osteoporosis and the spinal surgery field.
Osteoporosis is becoming a global problem, and as the size of the Asian geriatric population is rapidly increasing, so is the total number of osteoporotic patients. Osteoporosis is a condition characterized by low bone mineral density (BMD), micro-architectural deterioration of bony tissue, and a consequent increase in the risk of fracture, predominantly in the hip, spine, and forearm.123
The incidence of osteoporosis is higher in females, who typically have lower bone mass than males. Menopause leads to bone loss, with a loss of between 3% and 6% every year over a 5-year period. Thus, research regarding osteoporosis to date has primarily involved postmenopausal women.4
However in the spinal field, male osteoporosis is also important. Among those aged 50 years and older, the prevalence of osteopenia in Korea is 46.5% in males and 48.7% in females.5 Additionally, the prevalence of osteoporosis in spinal surgery patients older than 50 years was found to be 14.5% and 51.3% for males and females, respectively.6 Therefore, a reasonable proportion of patients with osteoporosis are male. Moreover, among osteoporosis patients in whom fractures occur, fracture-associated morbidity and mortality is reported to be higher in males than in females.7 Therefore, a study of male osteoporosis is important in the field of spinal surgery.
Academic interest in male osteoporosis has increased in recent years.8 There are clinical reports of an increased incidence of osteoporosis in elderly men8 and prostate cancer patients following orchiectomy,9 suggesting that androgen deprivation leads to osteoporosis.1011 An osteoporotic male animal model can be generated effectively in young rats,12 and although there are many reports comparing and analyzing the serum bone formation and resorption markers in aged male orchiectomized rats, there have been no published studies on young castrated male rats. Musculoskeletal maturity in rats is defined as no epiphyseal closure in the long bones, and a tapering off of skeletal growth occurs in male and female Sprague-Dawley rats at 28–32 weeks postnatal.13
We observed the changes in bone metabolism in young castrated male rats in order to determine the window of time during which osteoporosis can affect the management of spinal surgery and the mechanism of bone metabolism changes in males with osteoporosis.
Nine-week-old male Sprague-Dawley rats (n=39) were purchased and acclimated separately in pathogen-free ventilated cages with a controlled environment (temperature 22±4℃, humidity 65±5%, day-night cycle 06:00–18:00). The rats were permitted free intake of tap water and standard rodent chow (SAFE, Augy, France) containing 8 g/kg calcium, 4.2 g/kg phosphorus, and 1000 UI/kg vitamin D3.
After 1 week of housing, 15 rats were orchiectomized to induce male osteoporosis, and 15 underwent a sham operation. All rats in the orchiectomy (OCX) group underwent bilateral orchiectomy. Those in the sham surgery (sham) group underwent the same procedure, except that the testes were identified and then preserved.
At postoperative date (POD) 8, 10, and 12 weeks, five of 15 orchiectomized rats were euthanized, their femurs and lumbar spines were removed, and BMD was measured via micro computed tomography (CT) scan. Cardiac puncture was serially performed in three rats to obtain serum at POD 0, 1, 4, 8, and 12 weeks.
All rats were sacrificed using CO2 inhalation. This experimental protocol was approved by the Institutional Animal Care and Use Committee of the Clinical Research Center of our institute (Fig. 1).
Anesthesia was induced with 5% isoflurane, and Rompun (2.5 mg/kg, Bayer Korea, Seoul, Korea) and Zoletil (5 mg/kg, Virbac Korea, Seoul, Korea) were then injected intraperitoneally for generalized anesthesia. Maintenance of anesthesia was achieved by the administration of 2.5% isoflurane and oxygen via a coaxial nose cone. Bilateral orchiectomy was performed via a scrotal approach. The anesthetized rat was placed supine on the operating table, and its position was fixed using adhesive tape. The scrotal hair was bilaterally shaved, and betadine preparation was performed as an aseptic maneuver. If the cremaster muscle was stimulated during the betadine preparation, resulting in ascension of the testes, a downward stroke was performed to lower the testes back into place. A small 1.0-cm median incision was made through the skin at the tip of the scrotum. The cremaster muscles were opened with an incision. At the entrance to the scrotal cavity, the testicular fat pad was located with the testis, followed by the caput epididymis, the vas deferens, and the testicular blood vessels, all of which were pulled through the incision using blunt forceps. After identifying the testis, a single ligature was placed on the spermatic cord around the vas deferens and the blood vessels. The testis and epididymis were removed. This procedure was repeated for the other testis and epididymis. The cremaster muscle and scrotal skin were sutured layer by layer.
The same preparation was performed on animals in the sham operation group, allowing the authors to visually identify each testis, epididymis, vas deferens, and testicular blood vessels. After visual identification, the cremaster muscle and skin were sutured without ligation or resection.12
The rats were euthanized, and the femur and lumbar spine were obtained from each rat. BMD was measured in the femur and lumbar spine using micro CT (SkyScan1173, Bruker-CT, Kontich, Belgium) and NRecon (Ver. 1.6.9.4, Bruker, Kontich, Belgium) software. Pixel image dimensions were 2240×2240 pixels with a resolution of 27.70 µm. Areas of analysis comprised the L5 vertebral body and the femora, from the lower end plate proceeding 3 mm cranially and from the distal femora proceeding 3 mm proximally (Fig. 2).
Rats were maintained nil per os after midnight and placed in a single cage for ether anesthesia. Blood was collected via cardiac puncture in the morning under sufficient artificial light. Blood samples were clotted for 10 min at room temperature and then centrifuged for 15 min at 1000 g and 4℃. The serum was obtained, divided into several aliquots, and stored at -20℃.
Serum levels of N-terminal type 1 procollagen (P1NP), osteocalcin, and C-telopeptide (CTX)-1 were measured using ELISA kits (MyBioSource Inc., kit: MBS2506450, San Diego, CA, USA; Biomedical Technologies Inc., kit: BT-490, Stoughton, MA, USA; IDS Inc., kit: AC-06F1, Boldon, North-East England, UK). Serum samples were added to the appropriate micro ELISA plate wells and combined with the specific antibody. A biotinylated detection antibody specific to P1NP and Avidin-Horseradish Peroxidase (HRP) conjugate was then added to each micro plate well successively and incubated. Free components were washed away. The substrate solution was added to each well. Only the wells that contained P1NP, biotinylated detection antibody, and Avidin-HRP conjugate appeared blue in color. The enzyme-substrate reaction was terminated by the addition of a sulfuric acid solution, and the color turned yellow. The optical density (OD) was measured spectrophotometrically at a wavelength of 450 nm. The OD value was proportional to the concentration of P1NP. The concentrations of P1NP in the samples were calculated by comparing the OD of the samples with the standard curve. Serum osteocalcin and CTX measurement were performed in a similar manner using a different ELISA kit.
Serum total alkaline phosphatase (ALP) and bone-specific ALP activities were determined by protein electrophoresis. The blood serum was placed into liquid in a capillary tube and exposed to an electric current in order to separate the serum protein components into five major fractions by size and electrical charge. Protein electrophoresis was performed at Seoul Medical Science Institute, and Protein electrophoresis data were confirmed by a laboratory medicine specialist.
The results are presented as mean±standard deviation in the tables and as mean±standard error in the graphs. Statistical analysis was performed using SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA). The Friedman test was used to compare mean values of BMD within the same group, and the Mann-Whitney U test was used to compare values between the sham and OCX groups. A p-value of ≤0.05 was considered to be statistically significant.
There was a significant difference in BMD between the sham group and the OCX group. Femoral BMD measured in the sham and orchiectomy groups was 0.35±0.05 g/cm3 and 0.22±0.04 g/cm3, respectively, at POD 8 weeks, 0.43±0.07 g/cm3, and 0.29±0.02 g/cm3 at POD 10 weeks, and 0.35±0.04 g/cm3 and 0.26±0.01 g/cm3 at POD 12 weeks (Table 1, Figs. 3 and 5). BMD was measured at the fifth lumbar vertebra. There was an interesting difference in BMD between the sham group and the OCX group. At POD 8, 10, and 12 weeks, the BMD of the sham group was 0.62±0.04 g/cm3, 0.66±0.04 g/cm3, and 0.61±0.06 g/cm3 while that of the OCX group was 0.56±0.07 g/cm3, 0.56±0.03 g/cm3, and 0.49±0.04 g/cm3, respectively. At POD 10 and 12 weeks, there was a significant difference between the sham group and the OCX group (p=0.008, 0.008, respectively) (Table 2, Figs. 4 and 6).
Femoral bones showed a significant BMD decrease earlier than lumbar vertebrae, with a profound decrease of BMD at POD 8 weeks. A BMD increment in lumbar vertebrae was seen in young castrated adults, defined as 10 to 32 weeks of postnatal life at POD 8 and 10 weeks,131415 followed by a reduction in BMD at POD 12 weeks. Reduction in lumbar BMD was not as great as the reduction in femoral BMD at postoperative 8 weeks. Although no significant change in BMD was found in the transition from POD 8 weeks to 10 weeks, a profound decrease in BMD was found when transitioning from POD 10 weeks to POD 12 weeks. A statistically significant difference in BMD between POD 10 weeks and 12 weeks was found.
Young adult sham male rats reached peak BMD in the early stages; on the other hand, castrated male rats did not reach peak BMD during young adulthood (Figs. 3 and 4).
To assess the chemical factors associated with the changes in BMD, we measured serum levels of the markers osteocalcin, total and bone-specific ALP, P1NP as bone formation markers, and CTX as a bone resorption marker. Comparison between the OCX and sham groups at POD 12 weeks showed significant differences in serum bone markers P1NP, osteocalcin, and CTX (p=0.05); however, serum bone-specific and total ALP did not show significant differences (p=0.513 and 0.827, respectively). The OCX group showed elevated levels of bone markers (Table 3, Fig. 7).
Serial changes in serum bone markers of orchiectomized rats were observed. Serum levels of CTX and bone-specific and total ALP showed an abrupt decrease in the OCX group at POD 1 and 4 week, after which the levels increased, and the changes in serum CTX and bone-specific and total ALP were statistically significant (p=0.015, 0.014, 0.019, respectively). On the other hand, serum P1NP increased gradually, and serum osteocalcin showed a gradual increase and decrease (Table 4, Fig. 8).
In the area of spinal surgery, loss of bone parenchyma as a result of osteoporosis can result in instrumentation mal-location and failure of fusion.161718 We believe that this emphasizes the importance of evaluating the BMD of not only femoral bones but also lumbar bones for direct assessment of bone parenchyma. Sex hormones are recognized as important factors in the maintenance of bone mass and architecture.1920 Increased bone resorption is observed in women after menopause, whereas in males, the decline in gonadal function with age is far more progressive.821 We took this into account by analyzing the BMD of femoral and lumbar bones in a male osteoporosis rat model that involved orchiectomy to induce hypogonadism.
According to laboratory research reported so far, osteoporosis after orchiectomy occurs via three mechanisms: 1) a decrease in calcium reabsorption and an imbalance in bone metabolism following sex hormone deprivation can lead to lowered BMD, 2) an increase in receptor activators of nuclear factor kappa-B ligand (RANKL) in bone marrow (which upregulates free soluble RANKL in the bone marrow of aged rats) can stimulate bone metabolism,22 particularly increased bone turnover in association with increased serum ALP and osteocalcin levels, 2324 and 3) loss of spinal BMD after orchiectomy is correlated with a decrease in spinal blood flow.25
Preceding studies using male osteoporosis experimental models targeted aged orchiectomized male white rats23 and sought to analyze the antiresorptive and protective effects of medications such as atorvastatin, amlodipine, calcitonin, raloxifene, risedronate, green tea polyphenols, and soy protein with isoflavones on bones.1011262728 However, BMD loss after orchiectomy occurs only under exceptional circumstances of sex gland removal. When male rats were orchiectomized, several studies showed a statistically significant BMD reduction;1229 however, others showed minimal changes in BMD.30 Therefore, discussion is required regarding the time period when orchiectomy is performed and when osteoporosis might affect the management of spinal surgery and the mechanism of osteoporotic changes in castrated male animals. As we performed orchiectomy at the adolescent stage to observe active changes in hormonal balance, there were differences in the BMD changes and serum bone markers when compared with previous male castration experiments.20293031 The adolescent stage was determined according to the correlation of body weight with bony changes at different postnatal phases, and the transition from young adult to aged adult was defined as occurring between 8 and 32 weeks.131415
Considering our results that a statistically significant reduction in the BMD of femoral bones at POD 8 and 10 weeks is the point at which BMD begins to deteriorate in the lumbar spine, changes in BMD after sex gland resection may vary depending on the region of the skeletal system measured, and those changes in BMD of the lumbar spine may be slower than those of the more commonly used femoral bone. Additionally, it is notable that we identified the time at which significant changes in lumbar BMD occur in male osteoporosis animal models. Therefore, if one were to use the lumbar area of the orchiectomized male osteoporosis rat model, an observation period of more than 10 postoperative weeks may be required.
In previous studies, osteoporosis was reported to develop with high bone turnover,2329 in which bone resorption exceeds bone formation. In our study, at POD 12 weeks, increasing serum bone markers (serum osteocalcin, CTX, ALP, P1NP) in the OCX group revealed that osteoporotic changes occurring in young male rats with castration were related to high bone turnover.
Peak bone mass is related to later osteoporosis and fracture risk,32 and peak bone mass is typically achieved by the early-to-mid 20s in humans. In this study, castrated male rats did not reach peak BMD; on the other hand, sham male rats reached peak BMD during young adulthood. Serial changes in serum bone markers of castrated male rats that indicate a gradual decrease in bone metabolism and serial changes in BMD are relevant.
In conclusion, this experiment revealed the window of time during which osteoporosis affects the management of spinal surgery and the mechanism of bone metabolism changes in males with osteoporosis. Young castrated male rats have a reduction in femoral and lumbar BMD due to the mixture of two detrimental factors: young castrated male rats did not reach peak BMD, and increased bone turnover caused bone resorption to exceed bone formation. This study may contribute to a valuable model for studies on male osteoporosis and the spinal surgery field.
Figures and Tables
 | Fig. 1Time course and experimental groups. Thirty 10-week-old male rats were acclimated before the study. At POD 8, 10, and 12 weeks, five rats were euthanized, and their femurs and lumbar spines were removed for measurement of BMD. Cardiac puncture was performed in three rats to obtain serum at POD 0, 1, 4, 8, and 12 weeks serially (↑, time of serum bone marker measurements). *Statistically significant data. POD, postoperative date; Cp, cardiac puncture; OCX, orchiectomy; BMD, bone mineral density. |
 | Fig. 2BMD measurement using the NRecon software. BMD was measured in the femur and lumbar spine using micro computed tomography and NRecon software. BMD, bone mineral density. |
 | Fig. 3Changes in BMD of the femur. BMD is reported as g/cm3. Measured BMD was lowest at week 8 after OCX, with an increase to peak BMD at week 10 and a subsequent decrease at week 12. BMD, bone mineral density; OCX, orchiectomy; POD, postoperative date. |
 | Fig. 4Changes in BMD of the lumbar spine. BMD is reported as g/cm3. BMD of the OCX group decreased at POD 8, 10, and 12 weeks, and there was a statistically significant decrease at POD 10 and 12 weeks (*). BMD, bone mineral density; OCX, orchiectomy; POD, postoperative date. |
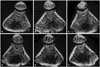 | Fig. 5Micro CT axial images of the femur. A, B, and C shows sham group rats at POD 8 weeks, 10 weeks, and 12 weeks. D, E, and F shows OCX group rats at POD 8 weeks, 10 weeks, and 12 weeks. POD, postoperative date; OCX, orchiectomy. |
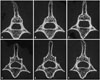 | Fig. 6Micro CT axial images of the lumbar spine. A, B, and C shows sham group rats at POD 8 weeks, 10 weeks, and 12 weeks. D, E, and F shows OCX group rats at POD 8 weeks, 10 weeks, and 12 weeks. POD, postoperative date; OCX, orchiectomy. |
 | Fig. 7Comparison of serum bone markers at POD 12 weeks. The OCX group showed elevated levels of bone markers, and P1NP, osteocalcin, and CTX showed statistically significant differences (*). POD, postoperative date; OCX, orchiectomy; P1NP, N-terminal type 1 procollagen; CTX, C-telopeptide. |
 | Fig. 8Serial changes in serum bone markers. (A) Bone formation markers. (B) Bone resorption marker. P1NP, N-terminal type 1 procollagen; ALP, alkaline phosphatase; OCX, orchiectomy; CTX-1, C-telopeptide-1. |
Table 1
BMD Value of Femur
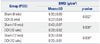
| Group (POD) | BMD (g/cm3) | |
|---|---|---|
| Mean±SD | p value | |
| Sham (8 wks) | 0.35±0.05 | 0.032* |
| OCX (8 wks) | 0.22±0.04 | |
| Sham (10 wks) | 0.43±0.07 | 0.008* |
| OCX (10 wks) | 0.29±0.02 | |
| Sham (12 wks) | 0.35±0.04 | 0.008* |
| OCX (12 wks) | 0.26±0.01 | |
Table 2
BMD Value of Lumbar Spine
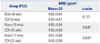
| Group (POD) | BMD (g/cm3) | |
|---|---|---|
| Mean±SD | p value | |
| Sham (8 wks) | 0.62±0.04 | 0.151 |
| OCX (8 wks) | 0.56±0.07 | |
| Sham (10 wks) | 0.66±0.04 | 0.008* |
| OCX (10 wks) | 0.56±0.03 | |
| Sham (12 wks) | 0.61±0.06 | 0.008* |
| OCX (12 wks) | 0.49±0.04 | |
Table 3
Levels of Serum Bone Markers

Table 4
Serial Changes in Serum Bone Markers

ACKNOWLEDGEMENTS
This work is financially supported by AOSpine Korea Research Grant 2016, application number AOSKR(R)2016-04.
References
1. Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med. 1991; 90:107–110.
2. Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011; 14:500–507.

3. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011; 377:1276–1287.

4. Ryu SJ, Yoo JS, Eom A, Koh SB, Choi JW. Roles of alendronate and simvastatin in prevention of bone loss in ovariectomized rats. Toxicol Environ Health Sci. 2011; 3:114–119.

5. Park EJ, Joo IW, Jang MJ, Kim YT, Oh K, Oh HJ. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2011. Yonsei Med J. 2014; 55:1049–1057.

6. Chin DK, Park JY, Yoon YS, Kuh SU, Jin BH, Kim KS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007; 18:1219–1224.

7. Kamel HK. Male osteoporosis: new trends in diagnosis and therapy. Drugs Aging. 2005; 22:741–748.
8. Blouin S, Gallois Y, Moreau MF, Baslé MF, Chappard D. Disuse and orchidectomy have additional effects on bone loss in the aged male rat. Osteoporos Int. 2007; 18:85–92.

10. Gradosova I, Zivna H, Palicka V, Hubena S, Svejkovska K, Zivny P. Protective effect of amlodipine on rat bone tissue after orchidectomy. Pharmacology. 2012; 89:37–43.

11. Gradosova I, Zivna H, Palicka V, Hubena S, Svejkovska K, Zivny P. Protective effect of atorvastatin on bone tissue in orchidectomised male albino Wistar rats. Eur J Pharmacol. 2012; 679:144–150.

12. Ryu SJ, Ryu DS, Kim JY, Park JY, Kim KH, Chin DK, et al. Bone mineral density changes after orchiectomy using a scrotal approach in rats. Korean J Spine. 2015; 12:55–59.

13. Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med. 2013; 4:624–630.
14. Sengupta P. A scientific review of age determination for a laboratory rat: how old is it in comparison with human age? Biomed Int. 2011; 2:81–89.
15. Agur AM, Dalley AF. Grant's atlas of anatomy. Philadelphia: Lippincott Williams & Wilkins;2009.
16. Coe JD, Warden KE, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants. A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine (Phila Pa 1976). 1990; 15:902–907.

17. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model: stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 2010; 48:309–312.

18. Park SB, Park SH, Kim NH, Chung CK. BMP-2 induced early bone formation in spine fusion using rat ovariectomy osteoporosis model. Spine J. 2013; 13:1273–1280.

19. Libouban H, Moreau MF, Legrand E, Audran M, Baslé MF, Chappard D. Comparison of histomorphometric descriptors of bone architecture with dual-energy X-ray absorptiometry for assessing bone loss in the orchidectomized rat. Osteoporos Int. 2002; 13:422–428.

20. Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Chung K, et al. Bone and mineral metabolism in the androgen-resistant (testicular feminized) male rat. J Bone Miner Res. 1993; 8:801–809.

21. Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab. 1997; 11:289–309.

22. Proell V, Xu H, Schüler C, Weber K, Hofbauer LC, Erben RG. Orchiectomy upregulates free soluble RANKL in bone marrow of aged rats. Bone. 2009; 45:677–681.

23. Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Bouillon R. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology. 1992; 130:2906–2916.

24. Khedr NF, El-Ashmawy NE, El-Bahrawy HA, Haggag AA, El-Abd EE. Modulation of bone turnover in orchidectomized rats treated with raloxifene and risedronate. Fundam Clin Pharmacol. 2013; 27:526–534.

25. Wang YX, Zhang YF, Griffith JF, Zhou H, Yeung DK, Kwok TC, et al. Vertebral blood perfusion reduction associated with vertebral bone mineral density reduction: a dynamic contrast-enhanced MRI study in a rat orchiectomy model. J Magn Reson Imaging. 2008; 28:1515–1518.

26. Al-Shahat AR, Shaikh MA, Elmansy RA, Shehzad K, Kaimkhani ZA. Prostatic assessment in rats after bilateral orchidectomy and calcitonin treatment. Endocr Regul. 2011; 45:29–36.
27. Juma SS, Ezzat-Zadeh Z, Khalil DA, Hooshmand S, Akhter M, Arjmandi BH. Soy protein with or without isoflavones failed to preserve bone density in gonadal hormone-deficient male rat model of osteoporosis. Nutr Res. 2012; 32:694–700.

28. Shen CL, Cao JJ, Dagda RY, Tenner TE Jr, Chyu MC, Yeh JK. Supplementation with green tea polyphenols improves bone microstructure and quality in aged, orchidectomized rats. Calcif Tissue Int. 2011; 88:455–463.

29. Broulik PD, Rosenkrancová J, Ruůzicka P, Sedlácek R. Effect of alendronate administration on bone mineral density and bone strength in castrated rats. Horm Metab Res. 2005; 37:414–418.

30. Wink CS, Felts WJ. Effects of castration on the bone structure of male rats: a model of osteoporosis. Calcif Tissue Int. 1980; 32:77–82.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download